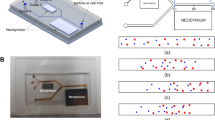
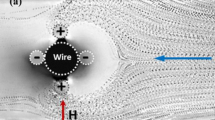
Abstract
Microfluidic separation technologies are the focus of various biological applications, such as disease diagnostics, single-cell analysis, and therapeutics. Different methods and devices were proposed in the micro-separation field, focusing on minimizing the chemical deformation and physical damage to the particles throughout the separation process; however, it is still a challenge. This paper proposes a hydrodynamic focusing-based microfluidic separation device equipped with a dual-neodymium magnet for positive magnetophoretic microparticles and cell separation. Hydrodynamic focusing is used to help to sort the particles and minimize the damage to the microparticles through the proposed different inlet flow rates between the two focusing channels. The dual magnets help to separate the particles in two stages. The system’s novelty is integrating the hydrodynamic focusing with the dual magnetics system, where the hydrodynamic focusing is with variable inlet flow rates. The performance of the proposed microfluidic particle separator is numerically assessed under various operating parameters, including the concentration of the particle in the injected solution and flow rate ratios of high to the low focusing flows on the efficiency of the separation. Following the proposed separation method, it was possible to separate the 16 and 10 \(\mu\mathrm{m}\) microparticles with the first-round efficiency of 21% with a quality of 92%, respectively. The developed particle separation system can significantly broaden its applications in a variety of biomedical research studies.
Graphical abstract









Similar content being viewed by others
Abbreviations
- a:
-
Spherical microparticle diameter (m)
- \({\mathrm{D}}_{\mathrm{h}}\) :
-
Denotes the hydraulic diameter of the flow channel (m)
- \({\mathrm{F}}_{\mathrm{D}}\) :
-
Hydrodynamic drag force (N)
- \({\mathrm{F}}_{\mathrm{L}}\) :
-
Inertia lift force (N)
- \({\mathrm{F}}_{\mathrm{m}}\) :
-
Magnetic force (N)
- \({\mathrm{f}}_{\mathrm{L}}\) :
-
Lift coefficient
- \({\mathrm{f}}_{\mathrm{D}}\) :
-
Drag coefficient
- h:
-
Height of a rectangular channel (m)
- H:
-
Magnetic field at the center of the microparticle (A.m−1)
- \({m}_{p}\) :
-
Mass of the microparticle (kg)
- \({N}_{tpd}\) :
-
Number of target particles that are damaged
- \({N}_{tpo}\) :
-
Number of target particles collected at the target outlet
- \({N}_{tpi}\) :
-
Number of target particles released at the inlet
- \({N}_{opo}\) :
-
Number of other particles collected at the target outlet
- \({\mathrm{Re}}_{\mathrm{c}}\) :
-
Local microchannel Reynolds number
- \({\mathrm{U}}_{\mathrm{m}}\) :
-
Mean flow velocity of the fluid carrying the particles (m.s−1)
- \({V}_{p}\) :
-
Particle volume (m3)
- \({\mathrm{v}}_{\mathrm{f}}\) :
-
Fluid velocity (m.s−1)
- \({v}_{p}\) :
-
Microparticle velocity (m.s−1)
- \(\mathrm{w}\) :
-
Width of a rectangular channel (m)
- \({\mathrm{x}}_{\mathrm{C}}\) :
-
The microparticle position within the cross-section of the microchannel
- \({\uprho }_{\mathrm{f}}\) :
-
The density of the fluid carrying the particles (kg.m−3)
- \({\upmu }_{\mathrm{f}}\) :
-
Dynamic viscosity of the fluid carrying the particles (Pa.s)
- \({\mu }_{o}\) :
-
Free space permeability (\(4\uppi \times {10}^{-7}{\mathrm{Hm}}^{-1}\))
- \({\eta }_{1}\) :
-
One round separation efficiency
- \(\psi\) :
-
Quality of the collection
- \(\vartheta\) :
-
Percentage of damaged particles
- \(\chi\) :
-
Magnetic susceptibility
- \({\chi }_{p}\) :
-
Particle magnetic susceptibility
- \({\chi }_{f}\) :
-
Fluid magnetic susceptibility
- HFC:
-
Hydrodynamics focusing channel
- UHFC:
-
Upper hydrodynamics focusing channel
- LHFC:
-
Lower hydrodynamics focusing channel
- HFCsI:
-
Hydrodynamics focusing channels intersection
References
Sajeesh P, Sen AK (2014) Particle separation and sorting in microfluidic devices: a review. Microfluid Nanofluidics 17:1–52. https://doi.org/10.1007/s10404-013-1291-9
Whitesides GM (2006) The origins and the future of microfluidics. Nature 442:368–373. https://doi.org/10.1038/nature05058
Suresh S, Spatz J, Mills JP, Micoulet A, Dao M, Lim CT, Beil M, Seufferlein T (2005) Connections between single-cell biomechanics and human disease states: gastrointestinal cancer and malaria. Acta Biomater 1:15–30. https://doi.org/10.1016/j.actbio.2004.09.001
Alshareef M, Metrakos N, Juarez Perez E, Azer F, Yang F, Yang X, Wang G (2013) Separation of tumor cells with dielectrophoresis-based microfluidic chip. Biomicrofluidics 7:011803. https://doi.org/10.1063/1.4774312
Vaziri A, Gopinath A (2008) Cell and biomolecular mechanics in silico. Nat Mater 7:15–23. https://doi.org/10.1038/nmat2040
Cranston H, Boylan C, Carroll G, Sutera S, Williamson JR, Gluzman I, Krogstad D (1984) Plasmodium falciparum maturation abolishes physiologic red cell deformability. Science (80-) 223:400–403. https://doi.org/10.1126/science.6362007
Link DR, Grasland-Mongrain E, Duri A, Sarrazin F, Cheng Z, Cristobal G, Marquez M, Weitz DA (2006) Electric control of droplets in microfluidic devices. Angew Chem Int Ed 45:2556–2560. https://doi.org/10.1002/anie.200503540
Bhagat AAS, Bow H, Hou HW, Tan SJ, Han J, Lim CT (2010) Microfluidics for cell separation. Med Biol Eng Comput 48:999–1014. https://doi.org/10.1007/s11517-010-0611-4
Doddabasavana G, PadmaPriya K, Nagabhushana K (2012) A review of recent advances in separation and detection of whole blood components, World J Sci Technol 2.5:05–09. https://scholar.google.com/scholar?hl=en&q=Doddabasavana+GB%2C+PadmaPriya+K%2C+Nagabhushana+K+%282012%29+A+review+of+recent+advances+in+separation+and+detection+of+whole+blood+components.&btnG=&as_sdt=1%2C16&as_sdtp=
Kersaudy-Kerhoas M, Dhariwal R, Desmulliez MPY (2008) Recent advances in microparticle continuous separation. IET Nanobiotechnol 2:1. https://doi.org/10.1049/iet-nbt:20070025
Gómez-Pastora J, Karampelas IH, Bringas E, Furlani EP, Ortiz I (2019) Numerical analysis of bead magnetophoresis from flowing blood in a continuous-flow microchannel: implications to the bead-fluid interactions. Sci Rep 9:7265. https://doi.org/10.1038/s41598-019-43827-x
Xu H, Dong B, Xu S, Xu S, Sun X, Sun J, Yang Y, Xu L, Bai X, Zhang S, Yin Z, Song H (2017) High purity microfluidic sorting and in situ inactivation of circulating tumor cells based on multifunctional magnetic composites. Biomaterials 138:69–79. https://doi.org/10.1016/j.biomaterials.2017.05.035
Zhou Y, Kumar DT, Lu X, Kale A, DuBose J, Song Y, Wang J, Li D, Xuan X (2015) Simultaneous diamagnetic and magnetic particle trapping in ferrofluid microflows via a single permanent magnet. Biomicrofluidics 9:044102. https://doi.org/10.1063/1.4926615
Zeng X, Deng J, Vedantam Y, Tzeng P, Xuan T-R (2013) Magnetic separation of particles and cells in ferrofluid flow through a straight microchannel using two offset magnets. J Magn Magn Mater 346:118–123
Zhu L, Lichlyter T, Haidekker DJ, Mao MA (2011) Analytical model of microfluidic transport of nonmagnetic particles in ferrofluids under the influence of a permanent magnet. Microfluid Nanofluidics 10:1233–1245
Zhu T, Cheng R, Lee SA, Rajaraman E, Eiteman MA, Querec TD, Unger ER, Mao L (2012) Continuous-flow ferrohydrodynamic sorting of particles and cells in microfluidic devices, Microfluid. Nanofluidics 13:645. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4587988/ Accessed Sep 23, 2019
Chen Q, Li D, Lin J, Wang M, Xuan X (2017) Simultaneous separation and washing of nonmagnetic particles in an inertial ferrofluid/water coflow. Anal Chem 89:6915–6920. https://doi.org/10.1021/acs.analchem.7b01608
Zhang J, Yan S, Yuan D, Zhao Q, Tan SH, Nguyen NT, Li W (2016) A novel viscoelastic-based ferrofluid for continuous sheathless microfluidic separation of nonmagnetic microparticles. Lab Chip 16:3947–3956. https://doi.org/10.1039/c6lc01007e
Kye HG, Park BS, Lee JM, Song MG, Song HG, Ahrberg CD, Chung BG (2019) Dual-neodymium magnet-based microfluidic separation device. Sci Rep 9:9502. https://doi.org/10.1038/s41598-019-45929-y
Zhang J, Yan S, Yuan D, Alici G, Nguyen N-T, EbrahimiWarkiani M, Li W (2016) Fundamentals and applications of inertial microfluidics: a review. Lab Chip 16:10–34. https://doi.org/10.1039/C5LC01159K
Zhou Y, Ma Z, Ai Y (2020) Dynamically tunable elasto-inertial particle focusing and sorting in microfluidics. Lab Chip 20:568–581. https://doi.org/10.1039/c9lc01071h
Segre G, Silberberg A (1961) Radial particle displacements in poiseuille flow of suspensions. Nat 189:209–210
Park S, Zhang Y, Wang TH, Yang S (2011) Continuous dielectrophoretic bacterial separation and concentration from physiological media of high conductivity. Lab Chip 11:2893–2900. https://doi.org/10.1039/c1lc20307j
Odenbach S (2002) Ferrofluids - magnetically controllable fluids and their applications. Springer-Verlag Berlin Heidelberg, vol 594. https://doi.org/10.1007/3-540-45646-5
Iiguni Y, Suwa M, Watarai H (2004) High-magnetic-field electromagnetophoresis of micro-particles in a capillary flow system. J Chromatogr A 1032:165–171. https://doi.org/10.1016/j.chroma.2003.10.134
Staben ME, Zinchenko AZ, Davis RH (2003) Motion of a particle between two parallel plane walls in low-Reynolds-number Poiseuille flow. Phys Fluids 15:1711–1733. https://doi.org/10.1063/1.1568341
Krishnan GP, Leighton DT (1995) Inertial lift on a moving sphere in contact with a plane wall in a shear flow articles you may be interested in. Phys Fluids 7:2538. https://doi.org/10.1063/1.868755
Ganatos P, Weinbaum S, Pfeffer R (1980) A strong interaction theory for the creeping motion of a sphere between plane parallel boundaries. Part 1. Perpendicular motion. J Fluid Mech 99:739–753. https://doi.org/10.1017/S0022112080000870
Munaz A, Shiddiky MJA, Nguyen NT (2018) Recent advances and current challenges in magnetophoresis based micro magnetofluidics. Biomicrofluidics 12. https://doi.org/10.1063/1.5035388
Zhu T, Cheng R, Liu Y, He J, Mao L (2014) Combining positive and negative magnetophoreses to separate particles of different magnetic properties. Microfluid Nanofluidics 17:973–982. https://doi.org/10.1007/s10404-014-1396-9
Pamme N, Wilhelm C (2006) Continuous sorting of magnetic cells via on-chip free-flow magnetophoresis. Lab Chip 6:974–980. https://doi.org/10.1039/b604542a
Child HW, Del Pino PA, De La Fuente JM, Hursthouse AS, Stirling D, Mullen M, McPhee GM, Nixon C, Jayawarna V, Berry CC (2011) Working together: the combined application of a magnetic field and penetratin for the delivery of magnetic nanoparticles to cells in 3D. ACS Nano 5:7910–7919. https://doi.org/10.1021/nn202163v
Bhuvanendran Nair Gourikutty S, Chang CP, Puiu PD (2016) Microfluidic immunomagnetic cell separation from whole blood. J Chromatogr B Anal Technol Biomed Life Sci 1011:77–88. https://doi.org/10.1016/j.jchromb.2015.12.016
Robert D, Pamme N, Conjeaud H, Gazeau F, Iles A, Wilhelm C (2011) Cell sorting by endocytotic capacity in a microfluidic magnetophoresis device. Lab Chip 11:1902–1910. https://doi.org/10.1039/c0lc00656d
COMSOL, What is COMSOL Multiphysics (2019) https://www.comsol.com/comsol-multiphysics. Accessed Aug 1 2010
Mark JE, Polymer Data Handbook, Oxford Univ. Press. (n.d.) https://global.oup.com/academic/product/polymer-data-handbook-9780195181012?cc=cl&lang=en&. Accessed Mar 26 2020
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Zareer, M. Tunable hydrodynamic focusing with dual-neodymium magnet-based microfluidic separation device. Med Biol Eng Comput 60, 47–60 (2022). https://doi.org/10.1007/s11517-021-02438-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-021-02438-3