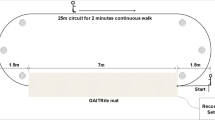
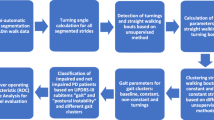
Abstract
Parkinson’s disease (PD) severity assessment in clinical settings largely depends on expertise level of clinicians which have inherent limitations and non-uniformity. Instrumented gait analysis plays a significant role in disease diagnosis and management. However, these are agonized from data dispersion due to demography, anthropometry, and self-selected walking speed of an individual. This research work aims to develop computationally efficient hybrid strategy using normalized features for PD severity evaluation. The relevance of each considered gait feature in demonstrating the outcomes is explained through feature importance and partial dependence plot (PDP) to build substantial insight for clinical needs. Gait, a biomarker, is used to access human mobility by utilizing vertical ground reaction force (VGRF) data of 72 healthy and 93 Parkinson’s individuals. A multi-variate normalization approach identifies gait differences between PD and non-PD. The proposed hybrid model used is able to detect PD with accuracy of 99.39% and 99.9%, and its severity assessment based on MDS-UPDRS-III shows coefficient of determination (R2) as 97% and 98.7% using leave-one-out cross-validation (CV) and tenfold CV respectively. The significant features suitable for clinical implications are reported. Moreover, normalized gait parameters supplement capability to compare individuals with diverse physical properties, resulting in assistive system for evaluation of PD severity.
Graphical abstract









Similar content being viewed by others
References
Hausdorff JM (2009) Gait dynamics in Parkinson’s disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos: An Interdisciplinary. Journal of Nonlinear Science 19(2): 026113
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376
Bhidayasiri R, Tarsy D (2012) Parkinson’s disease: Hoehn and Yahr scale. Movement disorders: a video atlas. Springer, pp 4–5
Goetz CG et al (2008) Movement disorder society-sponsored revision of the unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement disorders: official journal of the Movement Disorder Society 23(15):2129–2170
Rizzo G et al (2016) Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology 86(6):566–576
Hsu W-C et al (2018) Multiple-wearable-sensor-based gait classification and analysis in patients with neurological disorders. Sensors 18(10):3397
Joshi S, et al. (2010) Classification of Alzheimer’s disease and Parkinson’s disease by using machine learning and neural network methods. in 2010 Second International Conference on Machine Learning and Computing. 2010. IEEE
Amano S et al (2013) The effect of Tai Chi exercise on gait initiation and gait performance in persons with Parkinson’s disease. Parkinsonism Relat Disord 19(11):955–960
Pereira CR et al (2018) Handwritten dynamics assessment through convolutional neural networks: an application to Parkinson’s disease identification. Artif Intell Med 87:67–77
Sakar CO et al (2019) A comparative analysis of speech signal processing algorithms for Parkinson’s disease classification and the use of the tunable Q-factor wavelet transform. Appl Soft Comput 74:255–263
Balaji E, Brindha D, Balakrishnan R (2020) Supervised machine learning based gait classification system for early detection and stage classification of Parkinson’s disease. Applied Soft Computing, 94: p. 106494
El Maachi I, Bilodeau G-A, Bouachir W (2020) Deep 1D-convnet for accurate Parkinson disease detection and severity prediction from gait. Expert Systems with Applications 143: 113075
Khera P, Kumar N (2021) Age-gender specific prediction model for Parkinson’s severity assessment using gait biomarkers. Engineering Science and Technology, an International Journal
Tropea TF, Chen-Plotkin AS (2018) Unlocking the mystery of biomarkers: a brief introduction, challenges and opportunities in Parkinson disease. Parkinsonism Relat Disord 46:S15–S18
Wahid F et al (2016) A multiple regression approach to normalization of spatiotemporal gait features. J Appl Biomech 32(2):128–139
Morris ME (2006) Locomotor training in people with Parkinson disease. Phys Ther 86(10):1426–1435
Schwartz MH, Rozumalski A, Trost JP (2008) The effect of walking speed on the gait of typically developing children. J Biomech 41(8):1639–1650
Mullineaux DR et al (2006) Normalization of ground reaction forces. J Appl Biomech 22(3):230–233
Carty CP, Bennett MB (2009) The use of dimensionless scaling strategies in gait analysis. Hum Mov Sci 28(2):218–225
Aşuroğlu T et al (2018) Parkinson’s disease monitoring from gait analysis via foot-worn sensors. Biocybernetics and Biomedical Engineering 38(3):760–772
Frenkel-Toledo S et al (2005) Treadmill walking as an external pacemaker to improve gait rhythm and stability in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society 20(9):1109–1114
Hausdorff JM et al (2007) Rhythmic auditory stimulation modulates gait variability in Parkinson’s disease. Eur J Neurosci 26(8):2369–2375
Yogev G et al (2005) Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur J Neurosci 22(5):1248–1256
Perumal SV, Sankar R (2016) Gait and tremor assessment for patients with Parkinson’s disease using wearable sensors. Ict Express 2(4):168–174
Zhang Y, et al. (2013) Pathological gait detection of Parkinson’s disease using sparse representation. in 2013 International Conference on Digital Image Computing: Techniques and Applications (DICTA). IEEE
Ertuğrul ÖF et al (2016) Detection of Parkinson’s disease by shifted one dimensional local binary patterns from gait. Expert Syst Appl 56:156–163
Alkhatib R (2015) Gait-ground reaction force sensors selection based on ROC curve evaluation. J Comput Commun 3(03):13
Khoury N et al (2019) Data-driven based approach to aid Parkinson’s disease diagnosis. Sensors 19(2):242
Alam MN, et al. (2017) Vertical ground reaction force marker for Parkinson’s disease. PloS one, 2017. 12(5): e0175951
Zhao A et al (2018) A hybrid spatio-temporal model for detection and severity rating of Parkinson’s Disease from gait data. Neurocomputing 315:1–8
Wahid F et al (2015) Classification of Parkinson’s disease gait using spatial-temporal gait features. IEEE J Biomed Health Inform 19(6):1794–1802
Zeng W et al (2016) Parkinson’s disease classification using gait analysis via deterministic learning. Neurosci Lett 633:268–278
Prashanth R, Roy SD (2018) Novel and improved stage estimation in Parkinson’s disease using clinical scales and machine learning. Neurocomputing 305:78–103
Goldberger AL, et al. (2000) PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. circulation, 101(23): p. e215-e220
Sappakitkamjorn J, Niwitpong S-A (2013) Confidence intervals for the coefficients of variation with bounded parameters. International Journal of Mathematical and Computational Sciences 7(9):1416–1421
Rokach L, Maimon O (2005) Decision trees. Data mining and knowledge discovery handbook. Springer, pp 165–192
Elshawi R, Al-Mallah MH, Sakr S (2019) On the interpretability of machine learning-based model for predicting hypertension. BMC Med Inform Decis Mak 19(1):146
Molnar C (2020) Interpretable machine learning. Lulu. com. (PDP; Available as https://christophm.github.io/interpretable-ml-book/pdp.html)
Friedman JH (2001) Greedy function approximation: a gradient boosting machine. Annals of statistics, 1189–1232
Brown, J.D., Point-biserial correlation coefficients. Statistics, 2001. 5(3).
Acknowledgements
The authors are thankful to Director, CSIR-CSIO, Chandigarh for extending the necessary support. Khera P. expresses gratitude to University Grants Commission, India for supporting her Ph.D. through its national fellowship program. The authors acknowledge PhysioNet for database support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khera, P., Kumar, N. Novel machine learning-based hybrid strategy for severity assessment of Parkinson’s disorders. Med Biol Eng Comput 60, 811–828 (2022). https://doi.org/10.1007/s11517-022-02518-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-022-02518-y