Abstract
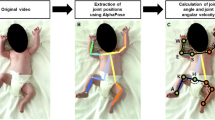
Early diagnosis of neurodevelopmental impairments in preterm infants is currently based on the visual analysis of newborns’ motion patterns by trained operators. To help automatize this time-consuming and qualitative procedure, we propose a sustainable deep-learning algorithm for accurate limb-pose estimation from depth images. The algorithm consists of a convolutional neural network (TwinEDA) relying on architectural blocks that require limited computation while ensuring high performance in prediction. To ascertain its low computational costs and assess its application in on-the-edge computing, TwinEDA was additionally deployed on a cost-effective single-board computer. The network was validated on a dataset of 27,000 depth video frames collected during the actual clinical practice from 27 preterm infants. When compared to the main state-of-the-art competitor, TwinEDA is twice as fast to predict a single depth frame and four times as light in terms of memory, while performing similarly in terms of Dice similarity coefficient (0.88). This result suggests that the pursuit of efficiency does not imply the detriment of performance. This work is among the first to propose an automatic and sustainable limb-position estimation approach for preterm infants. This represents a significant step towards the development of broadly accessible clinical monitoring applications.
Graphical abstract





Similar content being viewed by others
References
Turpin H, Urben S, Ansermet F, Borghini A, Murray MM, Müller-Nix C (2019) The interplay between prematurity, maternal stress and children’s intelligence quotient at age 11: a longitudinal study. Sci Rep 9(1):1–9
Gibbs R, Romero R, Hillier S, Eschenbach D, Sweet RL (1992) A review of premature birth and subclinical infection. Am J Obstet Gynecol 166(5):1515–1528
DeMaster D, Bick J, Johnson U, Montroy JJ, Landry S, Duncan AF (2019) Nurturing the preterm infant brain: leveraging neuroplasticity to improve neurobehavioral outcomes. Pediatr Res 85(2):166–175
Porro M, Fontana C, Giannì ML, Pesenti N, Boggini T, De Carli A, De Bon G, Lucco G, Mosca F, Fumagalli M et al (2020) Early detection of general movements trajectories in very low birth weight infants. Sci Rep 10(1):1–7
Fontana C, Ottaviani V, Veneroni C, Sforza SE, Pesenti N, Mosca F, Picciolini O, Fumagalli M, Dellacà RL (2021) Front Pediatr 868
Einspieler C, Prechtl HF, Ferrari F, Cioni G, Bos AF (1997) The qualitative assessment of general movements in preterm, term and young infants—review of the methodology. Early Hum Dev 50(1):47–60
Moccia S, Migliorelli L, Pietrini R, Frontoni E (2019) Preterm infants’ limb-pose estimation from depth images using convolutional neural networks. In: 2019 IEEE Conf Comput Intell Bioinforma Comput Biol. pp 1–7. https://doi.org/10.1109/CIBCB.2019.8791242
Moccia S, Migliorelli L, Carnielli V, Frontoni E (2020) Preterm infants’ pose estimation with spatio-temporal features. IEEE Trans Biomed Eng 67(8):2370–2380
Chen J, Ran X (2019) Deep learning with edge computing: a review. Proc IEEE 107(8):1655–1674
Cass S (2020) Nvidia makes it easy to embed AI: the Jetson Nano packs a lot of machine-learning power into DIY projects-[hands on]. IEEE Spectr 57(7):14–16
Migliorelli L, Moccia S, Pietrini R, Carnielli VP, Frontoni E (2020) The babyPose dataset. Data Brief 33(106):329
Strubell E, Ganesh A, McCallum A (2020) Energy and policy considerations for modern deep learning research. Proc AAAI Conf Artif Intel 34:13693–13696
Rashid M, Khan MA, Alhaisoni M, Wang SH, Naqvi SR, Rehman A, Saba T (2020) A sustainable deep learning framework for object recognition using multi-layers deep features fusion and selection. Sustainability 12(12):5037
Ascione R (2018) Il futuro della salute: come la tecnologia digitale sta rivoluzionando la medicina (e la nostra vita). Il futuro della salute, pp 1–270
Giovanola B, Tiribelli S (2022) Weapons of moral construction? On the value of fairness in algorithmic decision-making. Ethics Inf Technol 24(1):1–13
Fry KE, Chen YP, Howard A (2019) Discriminative models of spontaneous kicking movement patterns for term and preterm infants: a pilot study. IEEE Access 7:51357–51368
Airaksinen M, Räsänen O, Ilén E, Häyrinen T, Kivi A, Marchi V, Gallen A, Blom S, Varhe A, Kaartinen N et al (2020) Automatic posture and movement tracking of infants with wearable movement sensors. Sci Rep 10(1):1–13
Redd CB, Barber LA, Boyd RN, Varnfield M, Karunanithi MK (2019) Development of a wearable sensor network for quantification of infant general movements for the diagnosis of cerebral palsy. In: 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE, pp 7134–7139
Raghuram K, Orlandi S, Church P, Chau T, Uleryk E, Pechlivanoglou P, Shah V (2020) Automated movement recognition to predict motor impairment in high-risk infants: a systematic review of diagnostic test accuracy and meta-analysis. Dev Med Child Neurol
Miyagishima S, Asaka T, Kamatsuka K, Kozuka N, Kobayashi M, Igarashi L, Hori T, Tsutsumi H (2018) Spontaneous movements of preterm infants is associated with outcome of gross motor development. Brain Dev 40(8):627–633
Tsuji T, Nakashima S, Hayashi H, Soh Z, Furui A, Shibanoki T, Shima K, Shimatani K (2020) Markerless measurement and evaluation of general movements in infants. Sci Rep 10(1):1–13
Capio CM, Sit CH, Abernethy B, Masters RS (2012) Fundamental movement skills and physical activity among children with and without cerebral palsy. Res Dev Disabil 33(4):1235–1241
Marchi V, Hakala A, Knight A, D’Acunto F, Scattoni ML, Guzzetta A, Vanhatalo S (2019) Automated pose estimation captures key aspects of general movements at eight to 17 weeks from conventional videos. Acta Paediatr 108(10):1817–1824
Ihlen EA, Støen R, Boswell L, de Regnier RA, Fjørtoft T, Gaebler-Spira D, Labori C, Loennecken MC, Msall ME, Möinichen UI et al (2020) Machine learning of infant spontaneous movements for the early prediction of cerebral palsy: a multi-site cohort study. J Clin Med 9(1):5
McCay KD, Ho ES, Shum HP, Fehringer G, Marcroft C, Embleton ND (2020) Abnormal infant movements classification with deep learning on pose-based features. IEEE Access 8:51582–51592
Howard AG, Zhu M, Chen B, Kalenichenko D, Wang W, Weyand T, Andreetto M, Adam H (2017) Mobilenets: efficient convolutional neural networks for mobile vision applications. arXiv preprint arXiv:1704.04861
Ran X, Chen H, Zhu X, Liu Z, Chen J (2018) Deepdecision: a mobile deep learning framework for edge video analytics. In: IEEE Conference on Computer Communications. IEEE, pp 1421–1429
Wang F, Zhang M, Wang X, Ma X, Liu J (2020) Deep learning for edge computing applications: a state-of-the-art survey. IEEE Access 8:58322–58336
Lo SY, Hang HM, Chan SW, Lin JJ (2019) Efficient dense modules of asymmetric convolution for real-time semantic segmentation. In: Proceedings of the ACM Multimedia Asia. pp 1–6
Wang J, Xiong H, Wang H, Nian X (2020) ADSCNet: asymmetric depthwise separable convolution for semantic segmentation in real-time. Appl Intell 50(4):1045–1056
Chen LC, Zhu Y, Papandreou G, Schroff F, Adam H (2018) Encoder-decoder with atrous separable convolution for semantic image segmentation. In: European Conference on Computer Vision. pp 801–818
Huang G, Liu Z, Van DerMaaten L, Weinberger KQ (2017) Densely connected convolutional networks. In: IEEE Conference on Computer Vision and Pattern Recognition. pp 4700–4708
Adde L, Rygg M, Lossius K, Øberg GK, Støen R (2007) General movement assessment: predicting cerebral palsy in clinical practise. Early Hum Dev 83(1):13–18
Bulat A, Tzimiropoulos G (2016) Human pose estimation via convolutional part heatmap regression. In: European Conference on Computer Vision. Springer, pp 717–732
Fallang B, Saugstad OD, Grøgaard J, Hadders-Algra M (2003) Kinematic quality of reaching movements in preterm infants. Pediatr Res 53(5):836
van Wynsberghe A (2021) Sustainable AI: AI for sustainability and the sustainability of AI. AI Ethics 1–6
Schwartz R, Dodge J, Smith NA, Etzioni O (2020) Green AI. Commun ACM 63(12):54–63
Dhar P (2020) The carbon impact of artificial intelligence. Nat Mach Intell 2(8):423–425
Acknowledgements
The authors would like to acknowledge “L’Oréal Italia per le donne e la scienza” in collaboration with “Commissione Nazionale Italiana per l’UNESCO” which partially supported the project.
Funding
This work was supported by the European Union through the grants System Improvement for Neonatal Care (SINC) and SINC 2 under the EU POR FESR funding program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Migliorelli, L., Cacciatore, A., Ottaviani, V. et al. TwinEDA: a sustainable deep-learning approach for limb-position estimation in preterm infants’ depth images. Med Biol Eng Comput 61, 387–397 (2023). https://doi.org/10.1007/s11517-022-02696-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-022-02696-9