Abstract
Blood pressure (BP) is the main biomarker for monitoring patients, as its lack of control above values considered normal is a modifiable risk factor for target organ damage. The aim of this study is to evaluate the accuracy of the wearable electronic device photoplethysmography technology (PPG) Samsung Galaxy Watch 4 in determining BP in young patients compared to manual and automatic methods of BP determination. This is a quantitative and cross-sectional study, following validation protocols for wearable devices and BP measurement. It was carried out with twenty healthy young adults, in which BP was measured using four instruments, namely, standard sphygmomanometer device (manual), automatic arm oscillometric device (reference), wrist oscillometric device, and Smartwatch PPG. Eighty systolic blood pressure (SBP) and diastolic blood pressure (DBP) readings were observed. SBP means manual 118 ± 2.20,arm 113 ± 2.54, wrist 118 ± 2.51, and PPG (smartwatch) 113 ± 2.58. Among means, arm and PPG difference is 0.15, arm and wrist 4.95, arm and manual 4.45 wrist with PPG. The mean DBP manual 76.7 ± 1.84, arm 73.6 ± 1.92, wrist 79.3 ± 1.87, and PPG 72.2 ± 1.38. Among means, the difference between the arm and PPG is 1.4 and arm and hand 3.5 mmHg. The correlation shows PPG with manual, arm, and wrist. There was a strong SBP correlation and a moderate DBP correlation between the methods tested, evidencing the accuracy of the PPG smartwatch in relation to the reference method.
Graphical Abstract


Source: Own authorship, 2022. *p < 0.05, **p < 0.01, ***p < 0.001

Source: Own authorship, 2022. *p < 0.05, **p < 0.01, ***p < 0.001

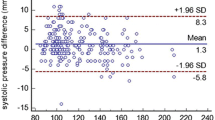
Source: Own authorship, 2022
Similar content being viewed by others
References
John O, Campbell NRC, Brady TM, Farrell M, Varghese C, Berumen AV et al (2021) The 2020 “WHO technical specifications for automated non-invasive blood pressure measuring devices with cuff.” Hypertension. 77:806–812
Mukkamala R, Hahn JO, Chandrasekhar A (2022) Photoplethysmography in noninvasive blood pressure monitoring. Photoplethysmography 359–400
Barroso WKS, Rodrigues CIS, Bortolotto LA, Mota-Gomes MA, Brandão AA, Feitosa ADM, Machado CA et al (2021) Brazilian guidelines of hypertension - 2020. Arq Bras Cardiol 116(3):516–658
Nilson EAF, Andrade RCS, Brito DA, Oliveira ML (2020) Costs attributable to obesity, hypertension and diabetes in the Unified Health System, Brazil, 2018. Rev Panam Salud Publica 44:e32
Charlton PH, Marozas V (2022) Wearable photoplethysmography devices. Photoplethysmography . 401–439
Brazil (2001) Resolution of the Collegiate Board (RDC) 185, of October 22, 2001. Approves the Technical Regulation contained in the annex to this Resolution, which deals with the registration, alteration, revalidation and cancellation of the registration of medical products with the National Health Surveillance Agency. Official Diary of the Union
Islam SMS, Chow CK, Daryabeygikhotbehsara R, Subedi N, Rawstorn J, Tegegne T et al (2022) Wearable cuffless blood pressure monitoring devices: a systematic review and meta-analysis. Eur Heart J Digital Health 3(2):323–337
Nelson BW, Low CA, Jacobson N, Areán P, Torous J, Allen NB (2020) Guidelines for wrist-worn consumer wearable assessment of heart rate in biobehavioral research. npj Digit Med 3(1):1–9
Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN et al (2005) Recommendations for blood pressure measurement in humans and experimental animals. Circulation 111(5):697–716
Lee HY, Lee DJ, Seo J, Ihm SH, Kim KI, Cho EJ et al (2021) Smartphone/smartwatch-based cuffless blood pressure measurement: a position paper from the Korean Society of Hypertension. Clin Hypertens 27(4):1–8
Colvonen PJ (2021) Response To: Investigating sources of inaccuracy in wearable optical heart rate sensors. npj Digit Med 4:38
Ware OR, Dawson JE, Shinohara MM, Taylor SC (2020) Racial limitations of fitzpatrick skin type. Cutis 105:77–80
Galindo GR, Mayer JA, Slymen D, Almaguer DD, Clapp E, Pichon LC et al (2007) Sun sensitivity in 5 US ethnoracial groups. Cutis 80:25
Pershing LK, Tirumala VP, Nelson JL, Corlett JL, Lin AG, Meyer LJ et al (2008) Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol 128:1633–1640
Stergiou GS, Alpert B, Mieke S, Asmar R, Atkins N, Eckert S et al (2018) A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J Hypertens 71(3):368–374
Bent B, Goldstein BA, Kibbe WA, Dunn JP (2020) Investigating sources of inaccuracy in wearable optical heart rate sensors. npj Digit Med 3(1):1–9
Peake JM, Kerr G, Sullivan JP (2018) A critical review of consumer wearables, mobile applications, and equipment for providing biofeedback, monitoring stress, and sleep in physically active populations. Front Physiol 9:743
Colvonen PJ (2021) Response To: Investigating sources of inaccuracy in wearable optical heart rate sensors. npj Digit Med 4(1):3–4
Boudreaux B (2018) Validity of wearable activity monitors during cycling and resistance exercise. Med Sci Sports Exerc 50(3):624–633
Shin G, Jarrahi MH, Fei Y, Karami A, Gafinowitz N, Byun A et al (2019) Wearable activity trackers, accuracy, adoption, acceptance and health impact: a systematic literature review. J Biomed Inform 93:103153
Malta DC, Bernal RTI, Prates EJS et al (2022) Self-reported arterial hypertension, use of health services and guidelines for care in Brazilian population: National Health Survey, 2019. Epidemiol Serv Saude 31(spe 1)
Cosoli G, Spinsante S, Scalise L (2020) Wrist-worn and chest-strap wearable devices: Systematic review on accuracy and metrological characteristics. Measurement 159:107789
Evenson KR, Goto MM, Furberg RD (2015) Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act 12(1)
Ng KG (2011) Review of measurement methods and clinical validation studies of noninvasive blood pressure monitors: Accuracy requirements and protocol considerations for devices that require patient-specific calibration by a secondary method or device before use. Blood Press Monitor 16(6):291–303
Peter L, Noury N, Cerny M (2014) A review of methods for non-invasive and continuous blood pressure monitoring: Pulse transit time method is promising? IRBM 35(5):271–282
Sharma M, Barbosa K, Ho V, Griggs D, Ghirmai T, Krishnan SK et al (2017) Cuff-less and continuous blood pressure monitoring: a methodological review. Technologies 5(2):21
Wang R (2017) Accuracy of wrist-worn heart rate monitors. JAMA Cardiol 2(1):104–106
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lins, L.F.T.d., do Nascimento, E.G.C., da Silva Júnior, J.A. et al. Accuracy of wearable electronic device compared to manual and automatic methods of blood pressure determination. Med Biol Eng Comput 61, 2627–2636 (2023). https://doi.org/10.1007/s11517-023-02869-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-023-02869-0