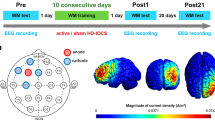
Abstract
High-frequency rTMS has been widely used to improve working memory (WM) impairment; however, the underlying neurophysiological mechanisms are unclear. We evaluated the effect of high-frequency rTMS on behaviors relevant to WM as well as coupling between theta and gamma oscillations in the prefrontal cortex (PFC) of rats. Accordingly, Wistar rats received high-frequency rTMS daily for 14 days (5 Hz, 10 Hz, and 15 Hz stimulation; 600 pulses; n = 6 per group), whereas the control group received sham stimulation. Electrophysiological signals were recorded simultaneously to obtain the local field potential (LFP) from the PFC, while the rats performed T-maze tasks for the evaluation of WM. Phase-amplitude coupling (PAC) was utilized to determine the effect of high-frequency rTMS on the theta-gamma coupling of LFPs. We observed that rats in the rTMS groups needed a smaller number of training days to complete the WM task as compared to the control group. High-frequency rTMS reinforced the coupling connection strength in the PFC of rats. Notably, the effect of rTMS at 15 Hz was the most effective among the three frequencies, i.e., 5 Hz, 10 Hz, and 15 Hz. The results suggested that rTMS can improve WM impairment in rats by modulating the coupling of theta and gamma rhythms. Hence, the current study provides a scientific basis for the optimization of TMS models, which would be relevant for clinical application.
Graphical abstract











Similar content being viewed by others
References
Stekic A, Zeljkovic M, Kontic MZ et al (2023) Intermittent theta burst stimulation ameliorates cognitive deficit and attenuates neuroinflammation via PI3K/Akt/mTOR signaling pathway in Alzheimer’s-like disease model. Alzheimer’s Dis Chall 16648714(2):44
Zangen A, Moshe H, Martinez D et al (2021) Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry 20(3):397–404
Chang CH, Liou MF, Liu CY et al (2022) Efficacy of repetitive transcranial magnetic stimulation in patients with methamphetamine use disorder: a systematic review and meta-analysis of double-blind randomized controlled trials. Front Psych 13:904252
Wei N, Chen J (2021) Repetitive transcranial magnetic stimulation for Alzheimer’s disease based on apolipoprotein E genotyping: protocol for a randomized controlled study. Front Aging Neurosci 13:758765
Gaertner M, Kong JT, Scherrer KH et al (2018) Advancing transcranial magnetic stimulation methods for complex regional pain syndrome: an open-label study of paired theta burst and high-frequency stimulation. Neuromodulation 21(4):409–416
Xu AH, Sun YX (2020) Research hotspots and effectiveness of repetitive transcranial magnetic stimulation in stroke rehabilitation. Neural Regen Res 15(11):2089
Guan M, Wang Z, Shi Y et al (2022) Altered brain function and causal connectivity induced by repetitive transcranial magnetic stimulation treatment for major depressive disorder. Front Neurosci 16:855483
Sverak T, Linhartova P, Gajdos M et al (2022) Brain connectivity and symptom changes after transcranial magnetic stimulation in patients with borderline personality disorder. Front Psych 12:2526
Yuan LQ, Zeng Q, Wang D et al (2021) Neuroimaging mechanisms of high-frequency repetitive transcranial magnetic stimulation for treatment of amnestic mild cognitive impairment: a double-blind randomized sham-controlled trial. Neural Regen Res 16(4):707
Yang S, Gao T, Wang J et al (2022) SAM: a unified self-adaptive multicompartmental spiking neuron model for learning with working memory. Front Neurosci 16:850945
Davis KE, Burnett K, Gigg J (2017) Water and T-maze protocols are equally efficient methods to assess spatial memory in 3xTg Alzheimer’s disease mice. Behav Brain Res 331:54–66
Nguyen JP, Suarez A, Kemoun G et al (2017) Repetitive transcranial magnetic stimulation combined with cognitive training for the treatment of Alzheimer’s disease. Neurophysiol Clin 47(1):47–53
Zhao J, Li Z, Cong Y et al (2017) Repetitive transcranial magnetic stimulation improves cognitive function of Alzheimer’s disease patients. Oncotarget 8(20):33864
Koch G, Bonnì S, Pellicciari MC et al (2018) Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage 169:302–311
Marra HLD, Myczkowski ML, Memória CM et al (2015) (2015) Transcranial magnetic stimulation to address mild cognitive impairment in the elderly: a randomized controlled study. Behav Neurol 2:1–13
Xue LY, Wu NN, Feng RR (2021) Research progress of protein phosphorylation in the regulation of synaptic plasticity. Chemistry of Life 41(4):686–692
Shang Y, Wang X, Shang X et al (2016) Repetitive transcranial magnetic stimulation effectively facilitates spatial cognition and synaptic plasticity associated with increasing the levels of BDNF and synaptic proteins in Wistar rats. Neurobiol Learn Mem 134:369–378
Wang F, Zhang Y, Wang L et al (2015) Improvement of spatial learning by facilitating large-conductance calcium-activated potassium channel with transcranial magnetic stimulation in Alzheimer’s disease model mice. Neuropharmacology 97:210–219
Riley MR, Qi XL, Zhou X et al (2018) Anterior-posterior gradient of plasticity in primate prefrontal cortex. Nat Commun 9(1):3790
Miller EK, Lundqvist M, Bastos AM (2018) Working memory 2.0. Neuron 100(2):463–475
He JW, Rabiller G, Nishijima Y et al (2020) Experimental cortical stroke induces aberrant increase of sharp-wave-associated ripples in the hippocampus and disrupts cortico-hippocampal communication. J Cereb Blood Flow Metab 40(9):1778–1796
Holmes CD, Papadimitriou C, Snyder LH (2018) Dissociation of LFP power and tuning in the frontal cortex during memory. J Neurosci 38(38):8177–8186
Chatzikalymniou AP, Skinner FK (2018). Deciphering the contribution of oriens-lacunosum/moleculare (OLM) cells to intrinsic θ rhythms using biophysical local field potential (LFP) models. Eneuro 5(4).
Morrone CD, Bazzigaluppi P, Beckett TL et al (2020) Regional differences in Alzheimer’s disease pathology confound behavioural rescue after amyloid-β attenuation. Brain 143(1):359–373
Colgin LL (2016) Rhythms of the hippocampal network. Nat Rev Neurosci 17(4):239–249
Buzsaki G, Draguhn A (2004) Neuronal oscillations in cortical networks. science 304(5679): 1926-1929
Roux F, Uhlhaas PJ (2014) Working memory and neural oscillations: alpha–gamma versus theta–gamma codes for distinct WM information? Trends Cogn Sci 18(1):16–25
Sullivan BJ, Ammanuel S, Kipnis PA et al (2020) Low-dose perampanel rescues cortical gamma dysregulation associated with parvalbumin interneuron GluA2 upregulation in epileptic syngap1+/− mice. Biol Psychiat 87(9):829–842
Nakazono T, Takahashi S, Sakurai Y (2019) Enhanced theta and high-gamma coupling during late stage of rule switching task in rat hippocampus. Neuroscience 412:216–232
Tamura M, Spellman TJ, Rosen AM et al (2017) Hippocampal-prefrontal theta-gamma coupling during performance of a spatial working memory task. Nat Commun 8(1):2182
Paxinos G, Watson C (2006) The rat brain in stereotaxic coordinates: hard, cover. Elsevier, Australia
Beynel L, Davis SW, Crowell CA et al (2019) Online repetitive transcranial magnetic stimulation during working memory in younger and older adults: a randomized within-subject comparison. PLoS One 14(3):e0213707
Tseng HJ, Lu CF, Jeng JS et al (2022) Frontal asymmetry as a core feature of major depression: a functional near-infrared spectroscopy study. J Psychiatry Neurosci 47(3):E186–E193
Zhang N, Xing M, Wang Y et al (2015) Repetitive transcranial magnetic stimulation enhances spatial learning and synaptic plasticity via the VEGF and BDNF–NMDAR pathways in a rat model of vascular dementia. Neuroscience 311:284–291
Xing H, Xiao Z, Qu R et al (2022) An efficient federated distillation learning system for multitask time series classification. IEEE Trans Instrum Meas 71:1–12
Xiao Z, Xu X, Xing H et al (2021) A federated learning system with enhanced feature extraction for human activity recognition. Knowl-Based Syst 229:107338
Biskamp J, Bartos M, Sauer JF (2017) Organization of prefrontal network activity by respiration-related oscillations. Sci Rep 7(1):1–11
Stevenson RF, Zheng J, Mnatsakanyan L et al (2018) Hippocampal CA1 gamma power predicts the precision of spatial memory judgments. Proc Natl Acad Sci 115(40):10148–10153
Spyropoulos G, Bosman CA, Fries P (2018) A theta rhythm in macaque visual cortex and its attentional modulation. Proc Natl Acad Sci 115(24):E5614–E5623
Canolty RT, Knight RT (2010) The functional role of cross-frequency coupling. Trends Cogn Sci 14(11):506–515
Nishida H, Takahashi M, Lauwereyns J (2014) Within-session dynamics of theta–gamma coupling and high-frequency oscillations during spatial alternation in rat hippocampal area CA1. Cogn Neurodyn 8:363–372
Lisman JE, Jensen O (2013) The theta-gamma neural code. Neuron 77(6):1002–1016
Jones KT, Johnson EL, Berryhill ME (2020) Frontoparietal theta-gamma interactions track working memory enhancement with training and tDCS. Neuroimage 211:116615
Welberg L (2014) Oscillations: synchrony shows mice the way. Nat Rev Neurosci 15(6):347
Cohen MX, Axmacher N, Lenartz D et al (2009) Good vibrations: cross-frequency coupling in the human nucleus accumbens during reward processing. J Cogn Neurosci 21(5):875–889
Zhang W, Guo L, Liu D et al (2020) The dynamic properties of a brain network during working memory based on the algorithm of cross-frequency coupling. Cogn Neurodyn 14:215–228
Alipour A, Mojdehfarahbakhsh A, Tavakolian A et al (2016) Neural communication through theta-gamma cross-frequency coupling in a bistable motion perception task. J Integr Neurosci 15(04):539–551
Friese U, Köster M, Hassler U et al (2013) Successful memory encoding is associated with increased cross-frequency coupling between frontal theta and posterior gamma oscillations in human scalp-recorded EEG. Neuroimage 66:642–647
Sun Y, Giacobbe P, Tang CW et al (2015) Deep brain stimulation modulates gamma oscillations and theta–gamma coupling in treatment resistant depression. Brain Stimul 8(6):1033–1042
Tort ABL, Kramer MA, Thorn C et al (2008) Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci 105(51):20517–20522
DeCoteau WE, Thorn C, Gibson DJ (2007) Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proc Natl Acad Sci 104(13):5644–5649
Lega B, Burke J, Jacobs J et al (2016) Slow-theta-to-gamma phase–amplitude coupling in human hippocampus supports the formation of new episodic memories. Cereb Cortex 26(1):268–278
Dayan E, Censor N, Buch ER et al (2013) Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci 16(7):838–844
Sale MV, Mattingley JB, Zalesky A et al (2015) Imaging human brain networks to improve the clinical efficacy of non-invasive brain stimulation. Neurosci Biobehav Rev 57:187–198
Xie J, Bai W, Liu T et al (2014) Functional connectivity among spike trains in neural assemblies during rat working memory task. Behav Brain Res 274:248–257
Douw L, Quaak M, Fitzsimmons SMD et al (2020) Static and dynamic network properties of the repetitive transcranial magnetic stimulation target predict changes in emotion regulation in obsessive-compulsive disorder. Brain Stimul 13(2):318–326
Seewoo BJ, Feindel KW, Etherington SJ et al (2019) Frequency-specific effects of low-intensity rTMS can persist for up to 2 weeks post-stimulation: a longitudinal rs-fMRI/MRS study in rats. Brain Stimul 12(6):1526–1536
Wirt RA, Hyman JM (2017) Integrating spatial working memory and remote memory: interactions between the medial prefrontal cortex and hippocampus. Brain Sci 7(4):43
Sapiurka M, Squire LR, Clark RE (2016) Distinct roles of hippocampus and medial prefrontal cortex in spatial and nonspatial memory. Hippocampus 26(12):1515–1524
Funding
This work was supported by the National Natural Science Foundation of China (Grant numbers 51737003, 51677053, and 51707054), the Natural Foundation of Hebei Province, China (Grant numbers E2021202222); the S&T Program of Hebei, China (Grant numbers 18963001D, 215676146H, 225676163GH); the Natural Science Foundation of Tianjin, China (Grant numbers 20JCQNJC00710, 21JCZXJC00090); and the State Key Laboratory of Reliability and Intelligence of Electrical Equipment of Hebei University of Technology (Grant number No.EERI_OY2021009).
Author information
Authors and Affiliations
Contributions
MG: developed the study concept, designed the study, and revised the manuscript. TW: data analysis and drafted and revised the manuscript. TZ: implementation of some experiments. HZ: data analysis. GX: developed the study concept. All authors approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Ethical statement
All the authors confirm that all animal experiments complied with the ARRIVE guidelines and were carried out in accordance with the UK Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments, or the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, M., Wang, T., Zhang, T. et al. Effects of high-frequency transcranial magnetic stimulation on theta-gamma oscillations and coupling in the prefrontal cortex of rats during working memory task. Med Biol Eng Comput 61, 3209–3223 (2023). https://doi.org/10.1007/s11517-023-02940-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-023-02940-w