Abstract
Purpose
Minimally invasive surgery is often built upon a time-consuming preoperative step consisting of segmentation and trajectory planning. At the temporal bone, a complete automation of these two tasks might lead to faster interventions and more reproducible results, benefiting clinical workflow and patient health.
Methods
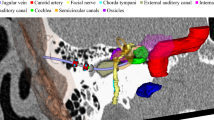
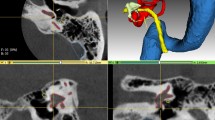
We propose an automatic segmentation and trajectory planning pipeline for image-guided interventions at the temporal bone. For segmentation, we use a shape regularized deep learning approach that is capable of automatically detecting even the cluttered tiny structures specific for this anatomy. We then perform trajectory planning for both linear and nonlinear interventions on these automatically segmented risk structures.
Results
We evaluate the usability of segmentation algorithms for planning access canals to the cochlea and the internal auditory canal on 24 CT data sets of real patients. Our new approach achieves similar results to the existing semiautomatic method in terms of Dice but provides more accurate organ shapes for the subsequent trajectory planning step. The source code of the algorithms is publicly available.
Conclusion
Automatic segmentation and trajectory planning for various clinical procedures at the temporal bone are feasible. The proposed automatic pipeline leads to an efficient and unbiased workflow for preoperative planning.












Similar content being viewed by others
References
Becker M, Kirschner M, Sakas G (2014) Segmentation of risk structures for otologic surgery using the probabilistic active shape model (pasm). Proc SPIE 9036:9036–7
Besl PJ, McKay ND (1992) A method for registration of 3-d shapes. IEEE Trans Pattern Anal Mach Int 14(2):239–256
Caversaccio M, Gavaghan K, Wimmer W, Williamson T, Ansò J, Mantokoudis G, Gerber N, Rathgeb C, Feldmann A, Wagner F, Scheidegger O, Kompis M, Weisstanner C, Zoka-Assadi M, Roesler K, Anschuetz L, Huth M, Weber S (2017) Robotic cochlear implantation: surgical procedure and first clinical experience. Acta Oto Laryngol 137(4):447–454
Cootes T, Taylor C, Cooper D, Graham J (1995) Active shape models-their training and application. Comput Vis Image Underst 61(1):38–59
Dahroug B, Tamadazte B, Weber S, Tavernier L, Andreff N (2018) Review on otological robotic systems: toward microrobot-assisted cholesteatoma surgery. IEEE Rev Biomed Eng 11:125–142
Fauser J, Stenin I, Kristin J, Klenzner T, Schipper J, Sakas G (2016) A software tool for planning and evaluation of non-linear trajectories for minimally invasive lateral skull base surgery. In: Tagungsb. der 15. Jahrestag. der Dtsch. Ges. f. Comput.- und Roboterass. Chirurgie e.V. (CURAC), pp 125–126
Fauser J, Sakas G, Mukhopadhyay A (2018) Planning nonlinear access paths for temporal bone surgery. Int J Comput Assist Radiol Surg 13(5):637–646
Fauser J, Stenin I, Kristin J, Klenzner T, Schipper J, Fellner D, Mukhopadhyay A (2018) Generalized trajectory planning for nonlinear interventions. In: OR 2.0 Context-Aware Operating Theaters, Computer Assisted Robotic Endoscopy, Clinical Image-Based Procedures, and Skin Image Analysis, Springer International Publishing, Cham, pp 46–53
Ferreira A, Tavares JMRS, Gentil F (2012) A review of segmentation algorithms for ear image data. In: 7th Iberian conference on information systems and technologies (CISTI 2012), pp 1–6
Fichera L, Dillon NP, Zhang D, Godage IS, Siebold MA, Hartley BI, Noble JH, Russell PT, Labadie RF, Webster RJ (2017) Through the eustachian tube and beyond: a new miniature robotic endoscope to see into the middle ear. IEEE Robot Autom Lett 2(3):1488–1494
Gerber N, Bell B, Gavaghan K, Weisstanner C, Caversaccio M, Weber S (2014) Surgical planning tool for robotically assisted hearing aid implantation. Int J Comput Assist Radiol Surg 9(1):11–20
Gerber N, Reyes M, Barazzetti L, Kjer HM, Vera S, Stauber M, Mistrik P, Ceresa M, Mangado N, Wimmer W, Stark T, Paulsen RR, Weber S, Caversaccio M, Ballester MAG (2017) A multiscale imaging and modelling dataset of the human inner ear. Sci Data 4:170132
Kirschner M (2013) The probabilistic active shape model: from model construction to flexible medical image segmentation. Ph.D. thesis, Technische Universität, Darmstadt
Kjer HM, Fagertun J, Vera S, Gil D, Ángel González Ballester M, Paulsen RR (2016) Free-form image registration of human cochlear mu ct data using skeleton similarity as anatomical prior. Pattern Recogn Lett 76:76–82 (special issue on Skeletonization and its application)
Labadie RF, Balachandran R, Noble JH, Blachon GS, Mitchell JE, Reda FA, Dawant BM, Fitzpatrick JM (2014) Minimally invasive image-guided cochlear implantation surgery: first report of clinical implementation. The Laryngoscope 124(8):1915–1922
Lorensen WE, Cline HE (1987) Marching cubes: a high resolution 3d surface construction algorithm. In: Proceedings of the 14th annual conference on computer graphics and interactive techniques, ACM, New York, NY, USA, SIGGRAPH ’87, pp 163–169
Lu P, Barazzetti L, Chandran V, Gavaghan K, Weber S, Gerber N, Reyes M (2018) Highly accurate facial nerve segmentation refinement from CBCT/CT imaging using a super-resolution classification approach. IEEE Trans Biomed Eng 65(1):178–188
Moghaddam B, Pentland A (1997) Probabilistic visual learning for object representation. IEEE Trans Pattern Anal Mach Intell 19(7):696–710
Noble JH, Dawant BM (2011) An atlas-navigated optimal medial axis and deformable model algorithm (nomad) for the segmentation of the optic nerves and chiasm in mr and ct images. Med Image Anal 15(6):877–884
Noble JH, Warren FM, Labadie RF, Dawant BM (2008) Automatic segmentation of the facial nerve and chorda tympani in ct images using spatially dependent feature values. Med Phys 35(12):5375–5384
Noble JH, Labadie RF, Majdani O, Dawant BM (2011) Automatic segmentation of intracochlear anatomy in conventional ct. IEEE Trans Biomed Eng 58(9):2625–2632
Powell KA, Liang T, Hittle B, Stredney D, Kerwin T, Wiet GJ (2017) Atlas-based segmentation of temporal bone anatomy. Int J Comput Assist Radiol Surg 12(11):1937–1944
Ronneberger O, Fischer P, Brox T (2015) U-Net: convolutional networks for biomedical image segmentation. Springer, Cham, pp 234–241
Ruiz Pujadas E, Piella G, Kjer HM, González Ballester MA (2018) Random walks with statistical shape prior for cochlea and inner ear segmentation in micro-ct images. Mach Vis Appl 29(3):405–414
Stenin I, Hansen S, Becker M, Sakas G, Fellner D, Klenzner T, Schipper J (2014) Minimally invasive multi-port surgery of the lateral skull base. BioMed Res Int 2014:7
Tack A, Mukhopadhyay A, Zachow S (2018) Knee menisci segmentation using convolutional neural networks: data from the osteoarthritis initiative. Osteoarthr Cartil 26(5):680–688
Torres R, Kazmitcheff G, De Seta D, Ferrary E, Sterkers O, Nguyen Y (2017) Improvement of the insertion axis for cochlear implantation with a robot-based system. Eur Arch Oto-Rhino-Laryngol 274(2):715–721
Voormolen E, van Stralen M, Woerdeman PA, Pluim JJ, Noordman H, Viergever MM, Regli L, van der Sprenkel JB (2012) Determination of a facial nerve safety zone for navigated temporal bone surgery. Neurosurgery 70(1):50
Weber S, Gerber N, Gavaghan KA, Williamson T, Wimmer W, Anso J, Brogna-Salas L, Chen D, Weisstanner C, Caversaccio M, Bell B (2013) Image guided and robotic assisted minimally invasive cochlear implantation. In: The Hamlyn symposium on medical robotics, pp 17–18
Xianfen D, Siping C, Changhong L, Yuanmei W (2005) 3d semi-automatic segmentation of the cochlea and inner ear. In: 2005 IEEE engineering in medicine and biology 27th annual conference, pp 6285–6288
Zhu S, Gao W, Zhang Y, Zheng J, Liu Z, Yuan G (2017) 3D automatic mri level set segmentation of inner ear based on statistical shape models prior. In: 2017 10th International congress on image and signal processing, biomedical engineering and informatics (CISP-BMEI), pp 1–6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This research was partially funded by the German Research Foundation. The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
This article is partially based on anonymized patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fauser, J., Stenin, I., Bauer, M. et al. Toward an automatic preoperative pipeline for image-guided temporal bone surgery. Int J CARS 14, 967–976 (2019). https://doi.org/10.1007/s11548-019-01937-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-019-01937-x