Abstract
Purpose
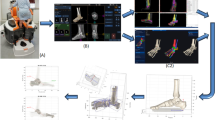
In the field of skeletal research, accurate and reliable segmentation methods are necessary for quantitative micro-CT analysis to assess bone quality. We propose a method of semi-automatic image segmentation of the midfoot, using the cuneiform bones as a model, based on thresholds set by phantom calibration that allows reproducible results in low cortical thickness bones.
Methods
Manual and semi-automatic segmentation methods were compared in micro-CT scans of the medial and intermediate cuneiforms of 24 cadaveric specimens. The manual method used intensity thresholds, hole filling, and manual cleanup. The semi-automatic method utilized calibrated bone and soft tissue thresholds Boolean subtraction to cleanly identify edges before hole filling. Intra- and inter-rater reliability was tested for the semi-automatic method in all specimens. Mask volume and average bone mineral density (BMD) were measured for all masks, and the three-dimensional models were compared to the initial semi-automatic segmentation using an unsigned distance part comparison analysis. Segmentation methods were compared with paired t-tests with significance level 0.05, and reliability was analyzed by calculating intra-class correlation coefficients.
Results
There were statistically significant differences in mask volume and BMD between the manual and semi-automatic segmentation methods in both bones. The intra- and inter-reliability was excellent for mask volume and bone density in both bones. Part comparisons showed a higher maximum distance between surfaces for the manual segmentation than the repeat semi-automatic segmentations.
Conclusion
We developed a semi-automatic micro-CT segmentation method based on calibrated thresholds. This method was designed specifically for use in bones with high rates of curvature and low cortical bone density, such as the cuneiforms, where traditional threshold-based segmentation is more challenging. Our method shows improvement over manual segmentation and was highly reliable, making it appropriate for use in quantitative micro-CT analysis.



Similar content being viewed by others
References
Boerckel JD, Mason DE, McDermott AM, Alsberg E (2014) Microcomputed tomography: approaches and applications in bioengineering. Stem Cell Res Ther 5(6):1–12. https://doi.org/10.1186/scrt534
Mao SS, Li D, Luo Y, Syed YS, Budoff MJ (2016) Application of quantitative computed tomography for assessment of trabecular bone mineral density, microarchitecture and mechanical property. Clin Imaging 40(2):330–338. https://doi.org/10.1016/j.clinimag.2015.09.016
Engelke K, Lang T, Khosla S, Qin L, Zysset P, Leslie WD, Shepherd JA, Schousboe JT (2015) Clinical use of quantitative computed tomography (QCT) of the hip in the management of osteoporosis in adults: the 2015 ISCD official positions—part I. J Clin Densitom 18(3):338–358. https://doi.org/10.1016/j.jocd.2015.06.012
Haidekker MA, Stevens HY, Frangos JA (2004) Computerized methods for X-ray-based small bone densitometry. Comput Methods Progr Biomed 73(1):35–42. https://doi.org/10.1016/S0169-2607(02)00164-5
Troy KL, Edwards WB (2018) Practical considerations for obtaining high quality quantitative computed tomography data of the skeletal system. Bone 110:58–65. https://doi.org/10.1016/j.bone.2018.01.013
Giambini H, Dragomir-Daescu D, Huddleston PM, Camp JJ, An K-N, Nassr A (2015) The effect of quantitative computed tomography acquisition protocols on bone mineral density estimation. J Biomech Eng. https://doi.org/10.1115/1.4031572
Sande EPS, Martinsen ACT, Hole EO, Olerud HM (2010) Interphantom and interscanner variations for Hounsfield units—establishment of reference values for HU in a commercial QA phantom. Phys Med Biol 55(17):5123–5135. https://doi.org/10.1088/0031-9155/55/17/015
Kalender WA, Felsenberg D, Genant HK, Fischer M, Dequeker J, Reeve J (1995) The European Spine Phantom—a tool for standardization and quality control in spinal bone mineral measurements by DXA and QCT. Eur J Radiol 20(2):83–92. https://doi.org/10.1016/0720-048X(95)00631-Y
Guo H, Song S, Wang J, Guo M, Cheng Y, Wang Y, Tamura S (2018) 3D surface voxel tracing corrector for accurate bone segmentation. Int J Comput Assist Radiol Surg 13(10):1549–1563. https://doi.org/10.1007/s11548-018-1804-9
Klein AA-O, Warszawski J, Hillengaß J, Maier-Hein KH (2018) Automatic bone segmentation in whole-body CT images. Int J Comput Assist Radiol Surg 14(1):21–29
Ben Younes L, Nakajima Y, Saito T (2014) Fully automatic segmentation of the Femur from 3D-CT images using primitive shape recognition and statistical shape models. Int J Comput Assist Radiol Surg 9(2):189–196. https://doi.org/10.1007/s11548-013-0950-3
McGrath H, Li P, Dorent R, Bradford R, Saeed S, Bisdas S, Ourselin S, Shapey J, Vercauteren T (2020) Manual segmentation versus semi-automated segmentation for quantifying vestibular schwannoma volume on MRI. Int J Comput Assist Radiol Surg. https://doi.org/10.1007/s11548-020-02222-y
Kurata Y, Nishio M, Kido A, Fujimoto K, Yakami M, Isoda H, Togashi K (2019) Automatic segmentation of the uterus on MRI using a convolutional neural network. Comput Biol Med 114:103438. https://doi.org/10.1016/j.compbiomed.2019.103438
Newton MD, Junginger L, Maerz T (2020) Automated microCT-based bone and articular cartilage analysis using iterative shape averaging and atlas-based registration. Bone 137:115417. https://doi.org/10.1016/j.bone.2020.115417
Rathnayaka K, Sahama T, Schuetz MA, Schmutz B (2011) Effects of CT image segmentation methods on the accuracy of long bone 3D reconstructions. Med Eng Phys 33(2):226–233. https://doi.org/10.1016/j.medengphy.2010.10.002
Pelt CE, Turner CM, Bachus KN, Bo Foreman K, Beals TC (2011) Micro-CT density analysis of the medial wall of the human medial cuneiform. Orthopedics 34(5):5–12. https://doi.org/10.3928/01477447-20110317-07
Palacio-Mancheno PE, Larriera AI, Doty SB, Cardoso L, Fritton SP (2014) 3D assessment of cortical bone porosity and tissue mineral density using high-resolution µCT: effects of resolution and threshold method. J Bone Miner Res 29(1):142–150. https://doi.org/10.1002/jbmr.2012
Baiker M, Milles J, Dijkstra J, Henning TD, Weber AW, Que I, Kaijzel EL, Löwik CWGM, Reiber JHC, Lelieveldt BPF (2009) Atlas-based whole-body segmentation of mice from low-contrast micro-CT data. Med Image Anal 14(6):723–737
Nicolielo LFP, Van Dessel J, Shaheen E, Letelier C, Codari M, Politis C, Lambrichts I, Jacobs R (2017) Validation of a novel imaging approach using multi-slice CT and cone-beam CT to follow-up on condylar remodeling after bimaxillary surgery. Int J Oral Sci 9(3):139–144
Xie W, Jacobs C, Charbonnier J, Ginneken BV (2020) Relational modeling for robust and efficient pulmonary lobe segmentation in CT scans. IEEE Trans Med Imaging. https://doi.org/10.1109/TMI.2020.2995108
Brehler M, Görres J, Vetter SY, Franke J, Grützner PA, Meinzer HP, Wolf I (2016) Intra-operative assessment of fractured articular surfaces in cone beam CT image data. Int J Comput Assist Radiol Surg 11:603–612. https://doi.org/10.1007/s11548-015-1304-0
Polak SJ, Candido S, Levengood SKL, Wagoner Johnson AJ (2012) Automated segmentation of micro-CT images of bone formation in calcium phosphate scaffolds. Comput Med Imaging Graph 36(1):54–65. https://doi.org/10.1016/j.compmedimag.2011.07.004
du Plessis A, Broeckhoven C, Guelpa A, le Roux SG (2017) Laboratory x-ray micro-computed tomography: a user guideline for biological samples. Gigascience 6(6):1–11. https://doi.org/10.1093/gigascience/gix027
Deuerling JM, Rudy DJ, Niebur GL, Roeder RK (2010) Improved accuracy of cortical bone mineralization measured by polychromatic microcomputed tomography using a novel high mineral density composite calibration phantom. Med Phys 37(9):5138–5145. https://doi.org/10.1118/1.3480507
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15(2):155–163. https://doi.org/10.1016/j.jcm.2016.02.012
Lenchik L, Heacock L, Weaver AA, Boutin RD, Cook TS, Itri J, Filippi CG, Gullapalli RP, Lee J, Zagurovskaya M, Retson T, Godwin K, Nicholson J, Narayana PA (2019) Automated segmentation of tissues using CT and MRI: a systematic review. Acad Radiol 26(12):1695–1706. https://doi.org/10.1016/j.acra.2019.07.006
Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK (2007) Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone 41(4):505–515. https://doi.org/10.1016/j.bone.2007.07.007
Verhelst P-J, Shaheen E, de Faria VK, Van der Cruyssen F, Shujaat S, Coudyzer W, Salmon B, Swennen G, Politis C, Jacobs R (2019) Validation of a 3D CBCT-based protocol for the follow-up of mandibular condyle remodeling. Dentomaxillofac Radiol 49(3):20190364. https://doi.org/10.1259/dmfr.20190364
Saha PK, Liang G, Elkins JM, Coimbra A, Duong LT, Williams DS, Sonka M (2011) A new osteophyte segmentation algorithm using partial shape model and its applications to rabbit femur anterior cruciate ligament transection via micro-CT imaging. IEEE Trans Biomed Eng. https://doi.org/10.1109/TBME.2011.2129519
Diederichs G, Link TM, Kentenich M, Schwieger K, Huber MB, Burghardt AJ, Majumdar S, Rogalla P, Issever AS (2009) Assessment of trabecular bone structure of the calcaneus using multi-detector CT: correlation with microCT and biomechanical testing. Bone 44:976–983. https://doi.org/10.1016/j.bone.2009.01.372
Commean PK, Ju T, Liu L, Sinacore DR, Hastings MK, Mueller MJ (2009) Tarsal and metatarsal bone mineral density measurement using volumetric quantitative computed tomography. J Digit Imaging 22(5):492–502. https://doi.org/10.1007/s10278-008-9118-z
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the Salt Lake City Veterans Affairs Medical Center.
Funding
There is no funding source.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Ethics approval
This study was approved by the Internal Institutional Review Board of the University of Utah (IRB #00071733).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Submitted to International Journal of Computer Assisted Radiology and Surgery October 2020
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Requist, M.R., Sripanich, Y., Peterson, A.C. et al. Semi-automatic micro-CT segmentation of the midfoot using calibrated thresholds. Int J CARS 16, 387–396 (2021). https://doi.org/10.1007/s11548-021-02318-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-021-02318-z