Abstract
Purpose
One in five women who undergo breast conserving surgery will need a second revision surgery due to remaining tumor. The iKnife is a mass spectrometry modality that produces real-time margin information based on the metabolite signatures in surgical smoke. Using this modality and real-time tissue classification, surgeons could remove all cancerous tissue during the initial surgery, improving many facets of patient outcomes. An obstacle in developing a iKnife breast cancer recognition model is the destructive, time-consuming and sensitive nature of the data collection that limits the size of the datasets.
Methods
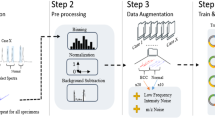
We address these challenges by first, building a self-supervised learning model from limited, weakly labeled data. By doing so, the model can learn to contextualize the general features of iKnife data from a more accessible cancer type. Second, the trained model can then be applied to a cancer classification task on breast data. This domain adaptation allows for the transfer of learnt weights from models of one tissue type to another.
Results
Our datasets contained 320 skin burns (129 tumor burns, 191 normal burns) from 51 patients and 144 breast tissue burns (41 tumor and 103 normal) from 11 patients. We investigate the effect of different hyper-parameters on the performance of the final classifier. The proposed two-step method performed statistically significantly better than a baseline model (p-value < 0.0001), by achieving an accuracy, sensitivity and specificity of 92%, 88% and 92%, respectively.
Conclusion
This is the first application of domain transfer for iKnife REIMS data. We showed that having a limited number of breast data samples for training a classifier can be compensated by self-supervised learning and domain adaption on a set of unlabeled skin data. We plan to confirm this performance by collecting new breast samples and extending it to incorporate other cancer tissues.





Similar content being viewed by others
References
World Cancer Research Fund. American Institute for Cancer Research, pages https://www.wcrf.org/dietandcancer/breast--cancer
Moran MS, Schnitt SJ, Guiliano AE, Harris JR, Khan SA, Horton J, Klimberg S, Chavez-MacGregor M, Freedman G, Houssami N, Johnson PL, Morrow M (2014) Society of surgical oncology-american society for radiation oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages i and ii invasive breast cancer. Clin Oncol 10:1507–1515
Maloney BW, McClatchy DM, Pogue BW, Paulsen KD, Wells WA, Barth RJ (2018) Review of methods for intraoperative margin detection for breast conserving surgery. J. Biomed. Optics. 23:1
Santilli A, Jamzad A, Janssen N, Kaufmann M, Connolly L, Vanderbeck K, Wang A, McKay D, Rudan J, Fichtinger G, Mousavi P (2020) Perioperative margin detection in bcc using a deep learning framework: a feasibility study. Int J CARS 15:887–96
Phelps DL, Balog J, Gildea LF, Bodai Z, Savage A, El-Bahrawy MA, Speller A, Rosini F, Kudo H, Brown R, Takats Z, G-Maghami S (2018) The surgical intelligent knife distinguishes normal, borderline and malignant gynaecological tissues using rapid evaporative ionisation mass spectrometry. British J Cancer 118:1349–58
Hanel L, Kwiatkowski M, Heikaus L, Schluter H (2019) Mass spectrometry-based intraoperative tumor diagnostics. Future Sci OA 5:FSO373
Noroozi M, Favaro P (2016) Unsupervised learning of visual representations by solving jigsaw puzzles. Eur. Conf Comput Vis 9910:69–84
Doersch C, Gupta A, Efros AA (2015) Unsupervised visual representation learning by context prediction. In: IEEE International Conference on Computer Vision, pp 1422–1430
Sermanet P, Lynch C, Chebotar Y, Hsu J, Jang E, Schaal S, Levine S (2017) Time-contrastive networks: Self-supervised learning from video. arXiv:1704.06888
Chung JS, Zisserman A (2017) Lip reading in profile. In: Proceedings of the British Machine Vision Conference, pp 1–11
Chen L, Bentley P, Mori K, Misawa K, Fujiwara M, Rueckert D (2019) Self-supervised learning for medical image analysis using image context restoration. Med Image Anal 58:101539
Maaten LVD, Hinton G (2008) Visualizing data using t-sne. J Machine Learn Res 9:2579–2605
St-John ER, Al-Khudairi R, Ashrafian H, Athanasiou T, Takats Z, Hadjiminas DJ, Darzi A, Leff DR (2017) Diagnostic accuracy of intraoperative techniques for margin assessment in breast cancer surgery. Analytical Surg 265(2):300–310
Santoro A, Drummond R, Silva I, Ferreira S, Juliano L, Vendramini P, da Costa Batista, Lemos M, Eberlin M, Andrade V (2020) In situ desi-msi lipidomic profiles of breast cancer molecular subtypes and precursor lesions. Cancer Res 80:1246–1257
Selvaraju RR, Cogswell M, Das A, Vedantam R, Parikh D, Batra D (2019) Grad-cam: Visual explanations from deep networks via gradient-based localization. Int J Comput Vis 128:336–359. https://doi.org/10.1007/s11263-019-01228-7
Funding
We would like to thank the following sources of funding: Natural Sciences and Engineering Council of Canada (NSERC), the Canadian Institute for Health Research (CIHR), Southeastern Ontario Academic Medical Organization (SEAMO) Innovation Fund, Britton Smith Chair in Surgery to J. Rudan, and Canada Research Chair to G. Fichtinger.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This study was approved by the Queen’s University Health Sciences Research Ethics Board.
Informed consent
All patients that participated in the study gave informed verbal and written consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santilli, A.M.L., Jamzad, A., Sedghi, A. et al. Domain adaptation and self-supervised learning for surgical margin detection. Int J CARS 16, 861–869 (2021). https://doi.org/10.1007/s11548-021-02381-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-021-02381-6