Abstract
Purpose
For tumor resections near critical structures, accurate identification of tumor boundaries and maximum removal are the keys to improve surgical outcome and patient survival rate, especially in neurosurgery. In this paper, we propose an intelligent optical diagnosis and treatment system for tumor removal, with automated lesion localization and laser ablation.
Methods
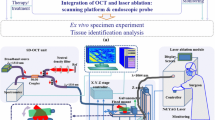
The proposed system contains a laser ablation module, an optical coherence tomography (OCT) unit, and a robotic arm along with a stereo camera. The robotic arm can move the OCT sample arm and the laser ablation front-end to the suspected lesion area. The corresponding diagnosis and treatment procedures include computer-aided lesion segmentation using OCT, automated ablation planning, and laser control. The ablation process is controlled by a deflectable mirror, and a non-common-path ablation planning algorithm based on the transformation from lesion positions to mirror deflection angles is presented.
Results
Phantom and animal experiments are carried out for system verification. The robot could reach the planned position with high precision, which is approximately 1.16 mm. Tissue classification with OCT images achieves 91.7% accuracy. The error of OCT-guided automated laser ablation is approximately 0.74 mm. Experiments on mouse brain tumors show that the proposed system is capable of clearing lesions efficiently and precisely. We also conducted an ex vivo porcine brain experiment to verify the whole process of the system.
Conclusion
An intelligent optical diagnosis and treatment system is proposed for tumor removal. Experimental results show that the proposed system and method are promising for precise and intelligent theranostics. Compared to conventional cancer diagnosis and treatment, the proposed system allows for automated operations monitored in real-time, with higher precision and efficiency.








Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Jolesz FA (2014) Intraoperative imaging and image-guided therapy. Springer, Berlin
Almeida JP, Chaichana KL, Rincon-Torroella J, Quinones-Hinojosa A (2015) The value of extent of resection of glioblastomas: clinical evidence and current approach. Curr Neurol Neurosci Rep 15(2):517. https://doi.org/10.1007/s11910-014-0517-x
Liao H, Noguchi M, Maruyama T, Muragaki Y, Kobayashi E, Iseki H, Sakuma I (2012) An integrated diagnosis and therapeutic system using intra-operative 5-aminolevulinic-acid-induced fluorescence guided robotic laser ablation for precision neurosurgery. Med Image Anal 16(3):754–766. https://doi.org/10.1016/j.media.2010.11.004
Liao H (2014) Integrated diagnostic and therapeutic techniques: toward an intelligent medical system. Comput Med Imaging Graph 38(5):421–422. https://doi.org/10.1016/j.compmedimag.2014.05.008
Lazarides AL, Whitley MJ, Strasfeld DB, Cardona DM, Ferrer JM, Mueller JL, Fu HL, Bartholf DeWitt S, Brigman BE, Ramanujam N, Kirsch DG, Eward WC (2016) A fluorescence-guided laser ablation system for removal of residual cancer in a mouse model of soft tissue sarcoma. Theranostics 6(2):155–166. https://doi.org/10.7150/thno.13536
Chen H, Zhao Y (2018) Applications of light-responsive systems for cancer theranostics. ACS Appl Mater Interfaces 10(25):21021–21034. https://doi.org/10.1021/acsami.8b01114
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA (1991) Optical coherence tomography. Science 254(5035):1178–1181. https://doi.org/10.1126/science.1957169
Wang J, Xu Y, Boppart SA (2017) Review of optical coherence tomography in oncology. J Biomed Opt 22(12):1–23. https://doi.org/10.1117/1.JBO.22.12.121711
Kut C, Chaichana KL, Xi J, Raza SM, Ye X, McVeigh ER, Rodriguez FJ, Quiñones-Hinojosa A, Li X (2015) Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci Transl Med 7(292):292ra100-292ra291. https://doi.org/10.1126/scitranslmed.3010611
Juarez-Chambi RM, Kut C, Rico-Jimenez JJ, Chaichana KL, Xi J, Campos-Delgado DU, Rodriguez FJ, Quinones-Hinojosa A, Li X, Jo JA (2019) AI-assisted in situ detection of human glioma infiltration using a novel computational method for optical coherence tomography. Clin Cancer Res Clincanres. https://doi.org/10.1158/1078-0432.ccr-19-0854
Dubey K, Singla N, Butola A, Lathe A, Quaiser D, Srivastava A, Mehta DS, Srivastava V (2019) Ensemble classifier for improve diagnosis of the breast cancer using optical coherence tomography and machine learning. Laser Phys Lett. https://doi.org/10.1088/1612-202X/aaf7ff
Luo S, Fan Y, Chang W, Liao H, Kang H, Huo L (2019) Classification of human stomach cancer using morphological feature analysis from optical coherence tomography images. Laser Phys Lett 16(9):095602. https://doi.org/10.1088/1612-202x/ab3638
Marvdashti T, Duan L, Aasi SZ, Tang JY, Ellerbee Bowden AK (2016) Classification of basal cell carcinoma in human skin using machine learning and quantitative features captured by polarization sensitive optical coherence tomography. Biomed Opt Express 7(9):3721. https://doi.org/10.1364/boe.7.003721
Zeng Y, Xu S, Chapman WC, Li S, Alipour Z, Abdelal H, Chatterjee D, Mutch M, Zhu Q (2020) Real-time colorectal cancer diagnosis using PR-OCT with deep learning. Theranostics 10(6):2587–2596. https://doi.org/10.7150/thno.40099
Yuan W, Kut C, Liang W, Li X (2017) Robust and fast characterization of OCT-based optical attenuation using a novel frequency-domain algorithm for brain cancer detection. Sci Rep 7(1):44909. https://doi.org/10.1038/srep44909
Fan Y, Xia Y, Zhang X, Sun Y, Tang J, Zhang L, Liao HE (2018) Optical coherence tomography for precision brain imaging, neurosurgical guidance and minimally invasive theranostics. Biosci Trends 12(1):12–23. https://doi.org/10.5582/bst.2017.01258
Boppart SA, Herrmann J, Pitris C, Stamper DL, Brezinski ME, Fujimoto JG (1999) High-resolution optical coherence tomography-guided laser ablation of surgical tissue. J Surg Res 82(2):275–284. https://doi.org/10.1006/jsre.1998.5555
Fan Y, Zhang B, Chang W, Zhang X, Liao H (2018) A novel integration of spectral-domain optical-coherence-tomography and laser-ablation system for precision treatment. Int J Comput Assist Radiol Surg 13(3):411–423. https://doi.org/10.1007/s11548-017-1664-8
Katta N, Estrada AD, McElroy AB, Gruslova A, Oglesby M, Cabe AG, Feldman MD, Fleming RD, Brenner AJ, Milner TE (2019) Laser brain cancer surgery in a xenograft model guided by optical coherence tomography. Theranostics 9(12):3555–3564. https://doi.org/10.7150/thno.31811
Maltais-Tariant R, Boudoux C, Uribe-Patarroyo N (2020) Real-time co-localized OCT surveillance of laser therapy using motion corrected speckle decorrelation. Biomedical Opt Express. https://doi.org/10.1364/boe.385654
Yuan W, Chen D, Sarabia-Estrada R, Guerrero-Cazares H, Li D, Quinones-Hinojosa A, Li X (2020) Theranostic OCT microneedle for fast ultrahigh-resolution deep-brain imaging and efficient laser ablation in vivo. Sci Adv 6(15):eaaz9664. https://doi.org/10.1126/sciadv.aaz9664
Garrido-Jurado S, Muñoz-Salinas R, Madrid-Cuevas FJ, Marín-Jiménez MJ (2014) Automatic generation and detection of highly reliable fiducial markers under occlusion. Pattern Recogn 47(6):2280–2292. https://doi.org/10.1016/j.patcog.2014.01.005
Fujimoto JG, Brezinski ME, Tearney GJ, Boppart SA, Bouma B, Hee MR, Southern JF, Swanson EA (1995) Optical biopsy and imaging using optical coherence tomography. Nat Med 1(9):970–972
Kim J, Brown W, Maher JR, Levinson H, Wax A (2015) Functional optical coherence tomography: principles and progress. Phys Med Biol 60(10):R211-237. https://doi.org/10.1088/0031-9155/60/10/R211
Guo S, Wei S, Lee S, Sheu M, Kang S, Kang JU (2019) Intraoperative speckle variance optical coherence tomography for tissue temperature monitoring during cutaneous laser therapy. IEEE J Transl Eng Health Med 7:1–8. https://doi.org/10.1109/jtehm.2019.2943317
Katta N, McElroy AB, Estrada AD, Milner TE (2018) Optical coherence tomography image-guided smart laser knife for surgery. Lasers Surg Med 50(3):202–212. https://doi.org/10.1002/lsm.22705
Fan Y, Sun Y, Chang W, Zhang X, Tang J, Zhang L, Liao H (2018) Bioluminescence imaging and two-photon microscopy guided laser ablation of GBM decreases tumor burden. Theranostics 8(15):4072–4085. https://doi.org/10.7150/thno.25357
LeCun Y, Jackel LD, Bottou L, Cortes C, Denker JS, Drucker H, Guyon I, Muller UA, Sackinger E, Simard P (1995) Learning algorithms for classification: a comparison on handwritten digit recognition. Neural Netw Stat Mech Perspect 261:276
Zhu M, Chang W, Jing L, Fan Y, Liang P, Zhang X, Wang G, Liao H (2019) Dual-modality optical diagnosis for precise in vivo identification of tumors in neurosurgery. Theranostics 9(10):2827–2842. https://doi.org/10.7150/thno.33823
Tsai RY, Lenz RK (1989) A new technique for fully autonomous and efficient 3 D robotics hand/eye calibration. IEEE Trans Robot Autom 5(3):345–358
Franz P, Wang X, Zhu H, Chia R, Hasenberg T, Wang H (2020) Detection of blackbody radiation during fiber guided laser-tissue vaporization. Biomed Opt Express 11(2):791–800. https://doi.org/10.1364/BOE.376141
Acknowledgements
The authors acknowledge supports from the National Natural Science Foundation of China (82027807, 81901907, 81771940) , and Beijing Municipal Natural Science Foundation (7212202).
Funding
The authors acknowledge supports from the National Natural Science Foundation of China (82027807, 81901907, 81771940), and Beijing Municipal Natural Science Foundation (7212202).
Author information
Authors and Affiliations
Contributions
Yangxi Li and Yingwei Fan contributed equally to this work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Ethics approval
The animal experiments in this study were approved by the Beijing Institute of Radiation Medicine Experiment Animal Center (Animal Protocols No. IACUC-DWZX-2019–502).
Consent to participate
This paper does not contain patient data.
Consent for publication
This paper does not contain patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yangxi Li and Yingwei Fan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, Y., Fan, Y., Hu, C. et al. Intelligent optical diagnosis and treatment system for automated image-guided laser ablation of tumors. Int J CARS 16, 2147–2157 (2021). https://doi.org/10.1007/s11548-021-02457-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-021-02457-3