Abstract
Purpose
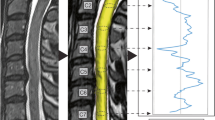
Spinal cord segmentation is the first step in atlas-based spinal cord image analysis, but segmentation of compressed spinal cords from patients with degenerative cervical myelopathy is challenging. We applied convolutional neural network models to segment the spinal cord from T2-weighted axial magnetic resonance images of DCM patients. Furthermore, we assessed the correlation between the cross-sectional area segmented by this network and the neurological symptoms of the patients.
Methods
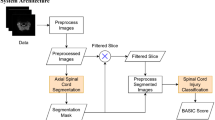
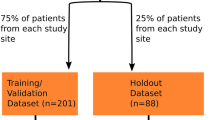
The CNN architecture was built using U-Net and DeepLabv3 + and PyTorch. The CNN was trained on 2762 axial slices from 174 patients, and an additional 517 axial slices from 33 patients were held out for validation and 777 axial slices from 46 patients for testing. The performance of the CNN was evaluated on a test dataset with Dice coefficients as the outcome measure. The ratio of CSA at the maximum compression level to CSA at the C2 level, as segmented by the CNN, was calculated. The correlation between the spinal cord CSA ratio and the Japanese Orthopaedic Association score in DCM patients from the test dataset was investigated using Spearman's rank correlation coefficient.
Results
The best Dice coefficient was achieved when U-Net was used as the architecture and EfficientNet-b7 as the model for transfer learning. Spearman's rs between the spinal cord CSA ratio and the JOA score of DCM patients was 0.38 (p = 0.007), showing a weak correlation.
Conclusion
Using deep learning with magnetic resonance images of deformed spinal cords as training data, we were able to segment compressed spinal cords of DCM patients with a high concordance with expert manual segmentation. In addition, the spinal cord CSA ratio was weakly, but significantly, correlated with neurological symptoms. Our study demonstrated the first steps needed to implement automated atlas-based analysis of DCM patients.






Similar content being viewed by others
References
Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG (2015) Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976) 40(12):E675–E693
Davies BM, Mowforth OD, Smith EK, Kotter MRN (2018) Degenerative cervical myelopathy. BMJ. https://doi.org/10.1136/bmj.k186
Evaniew N, Cadotte DW, Dea N, Bailey CS, Christie SD, Fisher CG, Paquet J, Soroceanu A, Thomas KC, Rampersaud YR, Manson NA, Johnson M, Nataraj A, Hall H, McIntosh G, Jacobs WB (2020) Clinical predictors of achieving the minimal clinically important difference after surgery for cervical spondylotic myelopathy: an external validation study from the Canadian Spine Outcomes and Research Network. J Neurosurg Spine SPI 33:129–137. https://doi.org/10.3171/2020.2.SPINE191495
De Leener B, Lévy S, Dupont SM, Fonov VS, Stikov N, Louis Collins D, Callot V, Cohen-Adad J (2017) SCT: spinal cord toolbox, an open-source software for processing spinal cord MRI data. Neuroimage 145:24–43. https://doi.org/10.1016/j.neuroimage.2016.10.009
Martin AR, De Leener B, Cohen-Adad J, Cadotte DW, Kalsi-Ryan S, Lange SF, Tetreault L, Nouri A, Crawley A, Mikulis DJ, Ginsberg H, Fehlings MG (2017) Clinically feasible microstructural MRI to quantify cervical spinal cord tissue injury using DTI, MT, and T2*-weighted imaging: assessment of normative data and reliability. Am J Neuroradiol 38:1257–1265. https://doi.org/10.3174/ajnr.A5163
McCoy DB, Talbott JF, Wilson M, Mamlouk MD, Cohen-Adad J, Wilson M, Narvid J (2017) MRI atlas-based measurement of spinal cord injury predicts outcome in acute flaccid myelitis. Am J Neuroradiol 38:410–417. https://doi.org/10.3174/ajnr.A5044
Hernandez ALCC, Rezende TJR, Martinez ARM, de Brito MR, França MCJ (2021) Tract-specific spinal cord diffusion tensor imaging in Friedreich’s Ataxia. Mov Disord. https://doi.org/10.1002/mds.28841
Long J, Shelhamer E, Darrell T (2015) Fully convolutional networks for semantic segmentation. In: Proc IEEE
De Leener B, Cohen-Adad J, Kadoury S (2015) Automatic segmentation of the spinal cord and spinal canal coupled with vertebral Labeling. IEEE Trans Med Imaging 34:1705–1718. https://doi.org/10.1109/TMI.2015.2437192
De Leener B, Kadoury S, Cohen-Adad J (2014) Robust, accurate and fast automatic segmentation of the spinal cord. Neuroimage 98:528–536. https://doi.org/10.1016/j.neuroimage.2014.04.051
Gros C, De Leener B, Badji A et al (2019) Automatic segmentation of the spinal cord and intramedullary multiple sclerosis lesions with convolutional neural networks. Neuroimage 184:901–915. https://doi.org/10.1016/j.neuroimage.2018.09.081
Sabaghian S, Dehghani H, Batouli SAH et al (2020) Fully automatic 3D segmentation of the thoracolumbar spinal cord and the vertebral canal from T2-weighted MRI using K-means clustering algorithm. Spinal Cord 58:811–820. https://doi.org/10.1038/s41393-020-0429-3
Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K (2001) Interobserver and intraobserver reliability of the Japanese Orthopaedic Association scoring system for evaluation of cervical compression myelopathy. Spine (Phila Pa 1976) 26:1890–1894. https://doi.org/10.1097/00007632-200109010-00014
Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K (1981) Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine (Phila Pa 1976). https://doi.org/10.1097/00007632-198107000-00005
Oshima Y, Takeshita K, Kato S, Doi T, Matsubayashi Y, Taniguchi Y, Nakajima K, Oguchi F, Okamoto N, Sakamoto R, Tanaka S (2020) Comparison between the Japanese orthopaedic association (JOA) score and patient-reported JOA (PRO-JOA) score to evaluate surgical outcomes of degenerative cervical myelopathy. Glob Spine J. https://doi.org/10.1177/2192568220964167
Ronneberger O, Fischer P, Brox T (2015) U-Net: convolutional networks for biomedical image segmentation. Medical image computing and computer-assisted intervention – MICCAI 2015. MICCAI 2015. Lecture notes in computer science. Springer, Cham
Chen L-C, Zhu Y, Papandreou G, Schroff F, Adam H (2018) Encoder-decoder with Atrous separable convolution for semantic image segmentation. Computer vision – ECCV 2018. ECCV 2018. Lecture notes in computer science. Springer, Cham
Altman DG, Bland TM (1983) Measurement in medicine: the analysis of method comparison Studies Author (s): DG Altman, JM Bland Published by: Wiley for the Royal Statistical Society Stable URL : http://www.jstor.org/stable/2987937 REFERENCES Linked references are ava. Statistician 32:307–317
Nakamura M, Fujiyoshi K, Tsuji O, Konomi T, Hosogane N, Watanabe K, Tsuji T, Ishii K, Momoshima S, Toyama Y, Chiba K, Matsumoto M (2012) Clinical significance of diffusion tensor tractography as a predictor of functional recovery after laminoplasty in patients with cervical compressive myelopathy. J Neurosurg Spine 17:147–152. https://doi.org/10.3171/2012.5.SPINE1196
Zhang X, Li Y, Liu Y, Tang SX, Liu X, Punithakumar K, Shi D (2021) Automatic spinal cord segmentation from axial-view MRI slices using CNN with grayscale regularized active contour propagation. Comput Biol Med 132:104345. https://doi.org/10.1016/J.COMPBIOMED.2021.104345
Ost K, Jacobs WB, Evaniew N, Cohen-adad J, Anderson D, Cadotte DW (2021) Spinal cord morphology in degenerative cervical myelopathy patients; Assessing key morphological characteristics using machine vision tools
McCoy DB, Dupont SM, Gros C, Cohen-Adad J, Huie RJ, Ferguson A, Duong-Fernandez X, Thomas LH, Singh V, Narvid J, Pascual L, Kyritsis N, Beattie MS, Bresnahan JC, Dhall S, Whetstone W, Talbott JF (2019) Convolutional neural network–based automated segmentation of the spinal cord and contusion injury: Deep learning biomarker correlates of motor impairment in acute spinal cord injury. Am J Neuroradiol 40:737–744. https://doi.org/10.3174/ajnr.A6020
Ito K, Yukawa Y, Machino M, Kato F (2013) Spinal cord cross-sectional area during flexion and extension in the patients with cervical ossification of posterior longitudinal ligament. Eur Spine J 22:2564–2568. https://doi.org/10.1007/s00586-013-2982-3
Ogino H, Tada K, Okada K, Yonenobu K, Yamamoto T, Ono K, Namiki H (1983) Canal diameter, anteroposterior compression ratio, and spondylotic myelopathy of the cervical spine. Spine (Phila Pa 1976) 8:1–15. https://doi.org/10.1097/00007632-198301000-00001
Fujiwara K, Yonenobu K, Hiroshima K, Ebara S, Yamashita K, Ono K (1988) Morphometry of the cervical spinal cord and its relation to pathology in cases with compression myelopathy. Spine (Phila Pa 1976) 13:1212–6. https://doi.org/10.1097/00007632-198811000-00002
Okada Y, Ikata T, Yamada H, Sakamoto R, Katoh S (1993) Magnetic resonance imaging study on the results of surgery for cervical compression myelopathy. Spine (Phila Pa 1976) 18:2024–2029. https://doi.org/10.1097/00007632-199310001-00016
Golash A, Birchall D, Laitt RD, Jackson A (2001) Significance of CSF area measurements in cervical spondylitic myelopathy. Br J Neurosurg 15:17–21. https://doi.org/10.1080/02688690020024337
Funaba M, Imajo Y, Suzuki H, Nishida N, Nagao Y, Sakamoto T, Sakai T (2020) The radiological characteristics associated with the development of myelopathy due to ossification of the posterior longitudinal ligaments at each responsible level based on spinal cord evoked potentials. Clin Neurol Neurosurg 194:105814. https://doi.org/10.1016/j.clineuro.2020.105814
Teresi LM, Lufkin RB, Reicher MA, Moffit BJ, Vinuela FV, Wilson GM, Bentson JR, Hanafee WN (1987) Asymptomatic degenerative disk disease and spondylosis of the cervical spine: MR imaging. Radiology 164:83–88. https://doi.org/10.1148/radiology.164.1.3588931
Maki S, Koda M, Ota M, Oikawa Y, Kamiya K, Inada T, Furuya T, Takahashi K, Masuda Y, Matsumoto K, Kojima M, Obata T, Yamazaki M (2018) Reduced field-of-view diffusion tensor imaging of the spinal cord shows motor dysfunction of the lower extremities in patients with cervical compression myelopathy. Spine (Phila Pa 1976) 43:89–96. https://doi.org/10.1097/BRS.0000000000001123
Martin AR, De Leener B, Cohen-Adad J, Cadotte DW, Nouri A, Wilson JR, Tetreault L, Crawley AP, Mikulis DJ, Ginsberg H, Fehlings MG (2018) Can microstructural MRI detect subclinical tissue injury in subjects with asymptomatic cervical spinal cord compression? A prospective cohort study. BMJ Open 8:e019809. https://doi.org/10.1136/bmjopen-2017-019809
Martin AR, De Leener B, Cohen-Adad J, Kalsi-Ryan S, Cadotte DW, Wilson JR, Tetreault L, Nouri A, Crawley A, Mikulis DJ, Ginsberg H, Massicotte EM, Fehlings MG (2018) Monitoring for myelopathic progression with multiparametric quantitative MRI. PLoS ONE 13:e0195733. https://doi.org/10.1371/journal.pone.0195733
Acknowledgements
The authors thank Ms. Sachi Yamashita for assisting with the preprocessing of the datasets.
Funding
This work was supported by JOA-Subsidized Science Project Research 2020–1, JSPS KAKENHI Grant Number JP20K18052.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
The study was approved by the local institutional review board of the Graduate School of Medicine, Chiba University. All procedures involving human participants were in accordance with the 1964 Declaration of Helsinki and its later amendments.
Informed consent
The requirement for informed consent was waived by the local institutional review board of the Graduate School of Medicine, Chiba University, because of the retrospective nature of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nozawa, K., Maki, S., Furuya, T. et al. Magnetic resonance image segmentation of the compressed spinal cord in patients with degenerative cervical myelopathy using convolutional neural networks. Int J CARS 18, 45–54 (2023). https://doi.org/10.1007/s11548-022-02783-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-022-02783-0