Abstract
Purpose
The purpose of this study was to assess if radiologists assisted by deep learning (DL) algorithms can achieve diagnostic accuracy comparable to that of pre-surgical biopsies in benign–malignant differentiation of musculoskeletal tumors (MST).
Methods
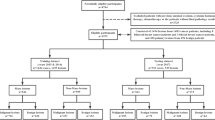
We first conducted a systematic review of literature to get the respective overall diagnostic accuracies of fine-needle aspiration biopsy (FNAB) and core needle biopsy (CNB) in differentiating between benign and malignant MST, by synthesizing data from the articles meeting our inclusion criteria. To compared against the accuracies reported in literature, we then invited 4 radiologists, respectively with 2 (A), 6 (B), 7 (C), and 33 (D) years of experience in interpreting musculoskeletal MRI to perform diagnostic tests on our own dataset (n = 62), with and without assistance of a previously developed DL algorithm. The gold standard for benign–malignant differentiation was histopathologic confirmation or clinical/radiographic follow-up.
Results
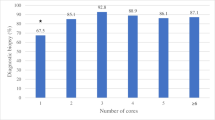
For FNAB, a meta-analysis containing 4604 samples met the inclusion criteria, with the overall diagnostic accuracy reported to be 0.77. For CNB, an overall accuracy of 0.86 was derived by synthesizing results from 7 original research articles containing a total of 587 samples. On our internal MST dataset, the invited radiologists, respectively, achieved diagnostic accuracies of 0.84 (A), 0.89 (B), 0.87 (C), and 0.90 (D), with the assistance of DL.
Conclusion
Use of DL algorithms on musculoskeletal dynamic contrast-enhanced MRI improved the benign–malignant differentiation accuracy of radiologists to a level comparable to that of pre-surgical biopsies. The developed DL algorithms have a potential to lower the risk of miss-diagnosing malignancy in radiological practice.



Similar content being viewed by others
References
WHO Classification of tumors Editorial Board (2020) WHO classification of tumors of soft tissue and bone, 5th edn. IARC Press, Lyon
Ferrari A, Dirksen U, Bielack S (2016) Sarcomas of soft tissue and bone. Prog Tumor Res 43:128–141. https://doi.org/10.1159/000447083
He Y, Pan I, Bao B, Halsey K, Chang M, Liu H, Peng S, Sebro RA, Guan J, Yi T, Delworth AT, Eweje F, States LJ, Zhang PJ, Zhang Z, Wu J, Peng X, Bai HX (2020) Deep learning-based classification of primary bone tumors on radiographs: a preliminary study. EBioMedicine 62:103121. https://doi.org/10.1016/j.ebiom.2020.103121
Widhe B, Widhe T (2000) Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am 82(5):667–674. https://doi.org/10.2106/00004623-200005000-00007
Wilson DAJ, Gazendam A, Visgauss J, Perrin D, Griffin AM, Chung PW, Catton CN, Shultz D, Ferguson PC, Wunder JS (2020) Designing a rational follow-up schedule for patients with extremity soft tissue sarcoma. Ann Surg Oncol 27(6):2033–2041. https://doi.org/10.1245/s10434-020-08240-z
Dodin G, Salleron J, Jendoubi S, Abou Arab W, Sirveaux F, Blum A, Gondim Teixeira PA (2021) Added-value of advanced magnetic resonance imaging to conventional morphologic analysis for the differentiation between benign and malignant non-fatty soft-tissue tumors. Eur Radiol 31(3):1536–1547. https://doi.org/10.1007/s00330-020-07190-0
Shannon BA, Ahlawat S, Morris CD, Levin AS, Fayad LM (2021) Do contrast-enhanced and advanced MRI sequences improve diagnostic accuracy for indeterminate lipomatous tumors? Radiol Med. https://doi.org/10.1007/s11547-021-01420-1
Leithner A et al (2009) Biopsy of bone and soft tissue tumours: hints and hazards”. Recent results in cancer research. Fortschritte der Krebsforschung Progres dans les recherches sur le cancer 179:3–10. https://doi.org/10.1007/978-3-540-77960-5_1
Crenn V, Vezole L, Bouhamama A, Meurgey A, Karanian M, Marec-Bérard P, Gouin F, Vaz G (2021) Percutaneous core needle biopsy can efficiently and safely diagnose most primary bone tumors. Diagnostics (Basel) 11(9):1552. https://doi.org/10.3390/diagnostics11091552
Chambers M, O’Hern K, Kerr DA (2020) Fine-needle aspiration biopsy for the diagnosis of bone and soft tissue lesions: a systematic review and meta-analysis. J Am Soc Cytopathol 9(5):429–441. https://doi.org/10.1016/j.jasc.2020.05.012
Schwartz HS, Spengler DM (1997) Needle tract recurrences after closed biopsy for sarcoma: three cases and review of the literature. Ann Surg Oncol 4(3):228–236. https://doi.org/10.1007/BF02306615
Mankin HJ, Lange TA, Spanier SS (1982) The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am 64(8):1121–1127
Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, Thrun S (2017) Dermatologist-level classification of skin cancer with deep neural networks. Nature 542(7639):115–118. https://doi.org/10.1038/nature21056
Ting DSW, Cheung CY, Lim G, Tan GSW, Quang ND, Gan A, Hamzah H, Garcia-Franco R, San Yeo IY, Lee SY, Wong EYM, Sabanayagam C, Baskaran M, Ibrahim F, Tan NC, Finkelstein EA, Lamoureux EL, Wong IY, Bressler NM, Sivaprasad S, Varma R, Jonas JB, He MG, Cheng CY, Cheung GCM, Aung T, Hsu W, Lee ML, Wong TY (2017) Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA 318(22):2211–2223. https://doi.org/10.1001/jama.2017.18152
Liang J, Huang X, Hu H, Liu Y, Zhou Q, Cao Q, Wang W, Liu B, Zheng Y, Li X, Xie X, Lu M, Peng S, Liu L, Xiao H (2018) Predicting malignancy in thyroid nodules: radiomics score versus 2017 american college of radiology thyroid imaging. Reporting Data Syst Thyroid 28(8):1024–1033. https://doi.org/10.1089/thy.2017.0525
Thomas J, Haertling T (2020) AIBx, artificial intelligence model to risk stratify thyroid nodules. Thyroid 30(6):878–884. https://doi.org/10.1089/thy.2019.0752
Eweje FR, Bao B, Wu J, Dalal D, Liao WH, He Y, Luo Y, Lu S, Zhang P, Peng X, Sebro R, Bai HX, States L (2021) Deep learning for classification of bone lesions on routine MRI. EBioMedicine 68:103402. https://doi.org/10.1016/j.ebiom.2021.103402
Bien N, Rajpurkar P, Ball RL, Irvin J, Park A, Jones E, Bereket M, Patel BN, Yeom KW, Shpanskaya K, Halabi S, Zucker E, Fanton G, Amanatullah DF, Beaulieu CF, Riley GM, Stewart RJ, Blankenberg FG, Larson DB, Jones RH, Langlotz CP, Ng AY, Lungren MP (2018) Deep-learning-assisted diagnosis for knee magnetic resonance imaging: Development and retrospective validation of MRNet. PLoS Med 15(11):e1002699. https://doi.org/10.1371/journal.pmed.1002699
Zhao K, Zhang M, Xie Z, Yan X, Wu S, Liao P, Lu H, Shen W, Fu C, Cui H, Fang Q, Mei J (2021) Deep learning assisted diagnosis of musculoskeletal tumors based on contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging. https://doi.org/10.1002/jmri.28025
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM (2011) QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536. https://doi.org/10.7326/0003-4819-155-8-201110180-00009
Letson GD, Ward WG (1999) Appropriate follow-up of orthopaedic oncology patients. Instr Course Lect 48:603–606
R Development Core Team (2017) R: a language and environment for statistical computing. Version 3.4.2. Vienna: R Foundation for Statistical Computing; [cited 2018 Oct 26]. http://www.Rproject.org/
Hegde V, Burke ZDC, Park HY, Zoller SD, Johansen D, Kelley BV, Levine B, Motamedi K, Federman NC, Seeger LL, Nelson SD, Bernthal NM (2018) Is core needle biopsy reliable in differentiating between aggressive benign and malignant radiolucent bone tumors? Clin Orthop Relat Res 476(3):568–577. https://doi.org/10.1007/s11999.0000000000000062
Qi D, Zhao M, Hu T, Zhang G (2019) Diagnostic yield of percutaneous core needle biopsy in suspected soft tissue lesions of extremities. J Int Med Res 47(6):2598–2606. https://doi.org/10.1177/0300060519849294
Noebauer-Huhmann IM, Amann G, Krssak M, Panotopoulos J, Szomolanyi P, Weber M, Czerny C, Breitenseher M, Grabner G, Bogner W, Nemec S, Dominkus M, Funovics P, Windhager R, Trattnig S (2015) Use of diagnostic dynamic contrast-enhanced (DCE)-MRI for targeting of soft tissue tumour biopsies at 3T: preliminary results. Eur Radiol 25(7):2041–2048. https://doi.org/10.1007/s00330-014-3576-0
Nouh MR, Abu Shady HM (2014) Initial CT-guided needle biopsy of extremity skeletal lesions: diagnostic performance and experience of a tertiary musculoskeletal center. Eur J Radiol 83(2):360–365. https://doi.org/10.1016/j.ejrad.2013.10.012
Kiatisevi P, Thanakit V, Sukunthanak B, Boonthatip M, Bumrungchart S, Witoonchart K (2013) Computed tomography-guided core needle biopsy versus incisional biopsy in diagnosing musculoskeletal lesions. J Orthop Surg (Hong Kong) 21(2):204–208. https://doi.org/10.1177/230949901302100218
Joshi A, Magar SR, Chand P, Panth R, Khatri Chhetri BR (2013) Tru-cut biopsy as the initial method of tissue diagnosis in bone tumors with soft tissue extension. Indian J Orthop 47(2):195–199. https://doi.org/10.4103/0019-5413.108917
Seng C, Png W, Tan MH (2013) Accuracy of core needle biopsy for musculoskeletal tumours. J Orthop Surg (Hong Kong) 21(1):92–95. https://doi.org/10.1177/230949901302100123
Rochwerger A, Mattei JC (2018) Management of soft tissue tumors of the musculoskeletal system. Orthop Traumatol Surg Res 104(1S):S9–S17. https://doi.org/10.1016/j.otsr.2017.05.031
Steffner R (2014) Benign bone tumors. Cancer Treat Res 162:31–63. https://doi.org/10.1007/978-3-319-07323-1_3
Daley NA, Reed WJ, Peterson JJ (2017) Strategies for biopsy of musculoskeletal tumors. Semin Roentgenol 52(4):282–290. https://doi.org/10.1053/j.ro.2017.04.005
Leithner A, Maurer-Ertl W, Windhager R (2009) Biopsy of bone and soft tissue tumours: hints and hazards. Recent Results Cancer Res 179:3–10. https://doi.org/10.1007/978-3-540-77960-5_1
Errani C et al (2013) Current concepts in the biopsy of musculoskeletal tumors. Sci World J 2013:538152. https://doi.org/10.1155/2013/538152
Acknowledgements
This study is funded by the National Natural Science Foundation of China (NSFC, No. 61876109).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Data sharing statement
The source code of this study is available at https://github.com/ahmedbesbes/mrnet.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, K., Zhu, X., Zhang, M. et al. Radiologists with assistance of deep learning can achieve overall accuracy of benign–malignant differentiation of musculoskeletal tumors comparable with that of pre-surgical biopsies in the literature. Int J CARS 18, 1451–1458 (2023). https://doi.org/10.1007/s11548-023-02838-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-023-02838-w