Abstract
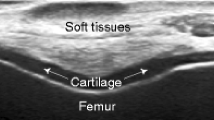
Articular cartilage is a thin and curvilinear structure whose image may get corrupted with noise during acquisition process. Image segmentation of this structure depends on expertise of an operator. In this paper, we propose an automated cartilage detection technique using 3D histogram and wavelet multiscale singularity analysis. 3D undecimated wavelet transform is implemented on MRI volume to obtain wavelet coefficients, which are used to determine an adaptive threshold for a given local resolution and a global threshold value. Local threshold helps to segment foreground cartilage edge details and is obtained using maximum likelihood estimate of the 3D histogram for a selected confidence level of intensity histogram. A global threshold is used to optimize coefficients using wavelet multiresolution singularity. The final wavelet coefficients are used to obtain a 3D model of cartilage tissue. The proposed method has been tested and validated using MRI and phantom datasets of articular cartilage. Quantitative analysis has been performed using mean square error (MSE), signal-to-noise ratio (SNR) and volumetric estimation of the datasets for different confidence and noise levels. The proposed method displays reduction in MSE for both denoised and noisy MRI volumes at different standard deviations of noise with overall improvement in SNR.





Similar content being viewed by others
References
Cicuttini, F.M., Forbes, A., Yuanyuan, W., Rush, G., Stuckey, S.L.: Rate of knee cartilage loss after partial meniscectomy. J. Rheumatol. 29(9), 1954–1956 (2002)
Kumar D., Hani A.F.M., Malik A., Kamil R., Razak R., Kiflie A.: Development of a non-invasive diagnostic tool for early detection of knee osteo arthritis. In: National postgraduate conference (NPC), pp. 1–6. (2011). doi:10.1109/NatPC.2011.6136338
Solloway, S., Hutchinson, C.E., Waterton, J.C., Taylor, C.J.: The use of active shape models for making thickness measurements of articular cartilage from MR images. Magn. Reson. Med. 37(6), 943–952 (1997)
Pham, D.L., Xu, C., Prince, J.L.: Current methods in medical image segmentation 1. Ann. Rev. Biomed. Eng. 2(1), 315–337 (2000)
Kapur T., Beardsley P., Gibson S., Grimson W., Wells W.: Model-based segmentation of clinical knee mri. In: Proceedings of IEEE international workshop on model-based 3D image analysis, Citeseer, pp. 97–106 (1998)
Hinrichs, E., Appleton, B., Lovell, B.C., Galloway, G.J.: Autonomous direct 3D segmentation of articular knee cartilage. In: Lovell, B.C. (ed.) Australian and New Zealand Intelligent Information Systems, vol. 1, pp. 417–420. Queensland University of Technology, Brisbane, Queensland (2003). http://espace.library.uq.edu.au/view/UQ:10702
Kass, M., Witkin, A., Terzopoulos, D.: Snakes: active contour models. Int. J. Comput. Vis. 1(4), 32–331 (1988)
Heimann, T., Meinzer, H.P.: Statistical shape models for 3D medical image segmentation: a review. Med. Image Anal. 13(4), 543–563 (2009)
Fripp J., Ourselin S., Warfield S., Mewes A., Crozier S.: Automatic generation of 3D statistical shape models of the knee bones. In: APRS Workshop on digital image computing, pp 15–21 (2005)
Baldwin, M.A., Langenderfer, J.E., Rullkoetter, P.J., Laz, P.J.: Development of subject-specific and statistical shape models of the knee using an efficient segmentation and mesh-morphing approach. Comput. Methods Programs Biomed. 97(3), 232–240 (2010)
Fripp, J., Crozier, S., Warfield, S.K., Ourselin, S.: Automatic segmentation and quantitative analysis of the articular cartilages from magnetic resonance images of the knee. IEEE Trans. Med. Imaging 29(1), 55–64 (2010)
Prasoon A., Petersen K., Igel C., Lauze F., Dam E., Nielsen M.: Deep feature learning for knee cartilage segmentation using a triplanar convolutional neural network. In: Medical Image Computing and Computer-Assisted Intervention-MICCAI 2013 (pp. 246–253). Springer, Berlin (2013)
Zhang, K., Lu, W., Marziliano, P.: Automatic knee cartilage segmentation from multi-contrast MR images using support vector machine classification with spatial dependencies. Magn. Reson. Imaging 31(10), 1731–1743 (2013)
Tamez-Pena, J.G., Farber, J., Gonzalez, P.C., Schreyer, E., Schneider, E., Totterman, S.: Unsupervised segmentation and quantification of anatomical knee features: data from the osteoarthritis initiative. IEEE Trans. Biomed. Eng. 59(4), 1177–1186 (2012)
Urish, K.L., Williams, A.A., Durkin, J.R., Chu, C.R.: Registration of magnetic resonance image series for knee articular cartilage analysis data from the osteoarthritis initiative. Cartilage 4(1), 20–27 (2013)
Withey, D.J., Pedrycz, W., Koles, Z.J.: Dynamic edge tracing: boundary identification in medical images. Comput. Vis. Image Underst. 113(10), 1039–1052 (2009)
Nain, D., Haker, S., Bobick, A., Tannenbaum, A.: Multiscale 3-d shape representation and segmentation using spherical wavelets. IEEE Trans. Med. Imaging 26(4), 598–618 (2007)
Pastor, L., Rodriguez, A., Espadero, J.M., Rincon, L.: 3D wavelet-based multiresolution object representation. Pattern Recognit. 34(12), 2497–2513 (2001)
Yin, Y., Zhang, X., Williams, R., Wu, X., Anderson, D.D., Sonka, M.: Logismos layered optimal graph image segmentation of multiple objects and surfaces: cartilage segmentation in the knee joint. IEEE Trans. Med. Imaging 29(12), 2023–2037 (2010)
Unser, M., Eden, M.: Multiresolution feature extraction and selection for texture segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 11(7), 717–728 (1989)
Amin, M.A., Yan, H.: High speed detection of retinal blood vessels in fundus image using phase congruency. Soft Comput. 15(6), 1217–1230 (2011)
Kovesi, P.: Phase congruency: a low level image invariant. Psychol. Res. 64(2), 136–148 (2000)
Gonzalez, R.C., Woods, R.E., Eddins, S.L.: Digital Image Using Matlab Processing. Person Prentice Hall, Lexington (2004)
Starck, J.L., Fadili, J., Murtagh, F.: The un-decimated wavelet decomposition and its reconstruction. IEEE Trans. Image Process. 16(2), 297–309 (2007)
Gudbjartsson, H., Patz, S.: The Rician distribution of noisy MRI data. Magn. Reson. Med. 34(6), 910–914 (2005)
Siddiqui M.: Statistical inference for rayleigh distributions. J. Res. Natl. Bur. Stand. Sec. D 68, 1005–1010 (1964)
Canavos, G.C.: Applied Probability and Statistical Methods. Little Brown, Boston (1984)
Forbes, C., Evans, M., Hastings, N., Peacock, B.: Statistical Distributions. Wiley, London (2011)
Wikipedia., Linear Interpolation. https://en.wikipedia.org/wiki/Linear_interpolation
Mallat, S., Hwang, W.L.: Singularity detection and processing with wavelets. IEEE Trans. Inf. Theory 38(2), 617–643 (1992)
Mallat, S., Zhong, S.: Characterization of signals from multi-scale edges. IEEE Trans. Pattern Anal. Mach. Intell. 14(7), 710–732 (1992)
Lun D.P.K., Hsung T.C., Ho Y.F.: Wavelet singularity detection for image processing. In: The 2002 45th Midwest Symposium on Circuits and Systems, 2002. MWSCAS-2002, IEEE, vol. 2, pp. II-56 (2002)
MathWorks., MATLAB R2013b. http://au.mathworks.com/products/new_products/release2013b.html
Aarya I., Jiang D., Gale T.: Adaptive rician denoising with edge preservation for mr images of the articular cartilage. In: Zhao, J. (ed.) Computer Methods in Biomechanics and Biomedical Engineering: Imaging and Visualization, pp. 1–10. Taylor and Francis (2014)
Schroeder, W., Lorenson, B., Martin, K.: The Visualization Toolkit, An Object-Oriented Approach to 3D Graphics, 3rd edn. Kitware Inc, New York (2004)
Myung, I.J.: Tutorial on maximum likelihood estimation. J. Math. Psychol. 47(1), 90–100 (2003)
Acknowledgments
We would like to thank Changai Ding and Menzies Institute of research, Tasmania, Australia, for providing us with the MRI datasets.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Rayleigh intensity interval estimation
To comput confidence interval for unknown mean \(\mu \) for confidence level \(\alpha 1\) for Rayleigh distribution, we assume the distribution to be sampled with respect to normal distribution.
where sample mean \(\mu _\mathrm{r}\) is obtained with unbiased estimator \(\hat{\sigma }\), which is obtained using MLE of Rayleigh probability distribution given by Eq. (5) [26, 27]. For N independent samples, the likelihood equation for the distribution is given as [26],
To obtain MLE for the distribution for unknown variance, take partial derivative with respect to \(\sigma ^{2}\) of the log-likelihood equation. The MLE for Rayleigh is given as,
Mean computed using this estimate is then given as,
To compute confidence limits using mean, rearrange the terms in the above Eq. (18)
where \( t_{1 - \alpha 1/2}\) can be obtained from the t-distribution table under given confidence level \(\alpha 1\) for \(N-1\) degrees of freedom.
Appendix 2: Gaussian intensity interval estimation
To compute confidence interval for Gaussian distribution with N independent observations for unknown mean, we use sampling for the given distribution from the normal distribution. The sampling distribution follows a t-distribution with \(N-1\) degree of freedom and is given as follows [27],
we determine the \(t_{(1 - \alpha 1/2 ),(N - 1)} \) value from the t-table for \(N - 1\) degree of freedom and is given as follows [27],
where \(\hat{X}\) is also the MLE for Gaussian distribution. Probability distribution for Gaussian distribution is given in Sect. 2 [27],
The likelihood equation for Gauss distribution with N independent samples is [36],
The MLE of the above equation with respect to mean is given as follows [36],
Appendix 3: Wavelet interscale difference
Wavelet coefficients are obtained by convolution of wavelet function on 2D image function I(x, y) as [23]
But image function I(x, y) follows a Rice probability distribution function given in Eq. (4) [25].
Thus wavelet coefficients obtained from the image are assumed to have a Rice distribution function, given as
We know in wavelet domain the modulus of sum of wavelet coefficients can be given as [32],
And the interscale difference between wavelet coefficients at two scale is given as,
But since the wavelet coefficients are governed by Rice probability distribution, the interscale difference is now given as,
Rights and permissions
About this article
Cite this article
Aarya, I., Jiang, D. Automated and optimal detection of 3D articular cartilage using undecimated wavelets in MRI. SIViP 9 (Suppl 1), 305–314 (2015). https://doi.org/10.1007/s11760-015-0825-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11760-015-0825-x