Abstract
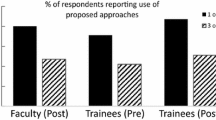
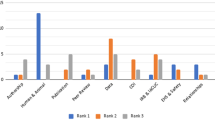
Training in the responsible conduct of research (RCR) is required for many research trainees nationwide, but little is known about its effectiveness. For a preliminary assessment of the effectiveness of a short-term course in RCR, medical students participating in an NIH-funded summer research program at the University of California, San Diego (UCSD) were surveyed using an instrument developed through focus group discussions. In the summer of 2003, surveys were administered before and after a short-term RCR course, as well as to alumni of the courses given in the summers of 2002 and 2001. Survey responses were analyzed in the areas of knowledge, ethical decision-making skills, attitudes about responsible conduct of research, and frequency of discussions about RCR outside of class. The only statistically significant improvement associated with the course was an increase in knowledge, while there was a non-significant tendency toward improvements in ethical decision-making skills and attitudes about the importance of RCR training. The nominal impact of a short-term training course should not be surprising, but it does raise the possibility that other options for delivering information only, such as an Internet-based tutorial, might be considered as comparable alternatives when longer courses are not possible.
Similar content being viewed by others
References
National Academy of Sciences (1992). Responsible science: Ensuring the integrity of the research process, Vol. I. Washington, D.C.: National Academies Press.
National Institutes of Health, and Alcohol, Drug Abuse, and Mental Health Administration (1989). Requirement for programs on the responsible conduct of research in National Research Service Award institutional training programs. NIH Guide for Grants and Contracts, 18, 1.
Bloom, B. S. (1956). Taxonomy of educational objectives: The classification of educational goals. New York, NY: David McKay Company, Inc.
Kalichman, M. W., & Friedman, P. J. (1992). A pilot study of biomedical trainees’ perceptions concerning research ethics. Academic Medicine, 67, 769–775.
Brown, S., & Kalichman, M. W. (1998) Effects of training in the responsible conduct of research: A survey of graduate students in experimental sciences. Science and Engineering Ethics, 4(4), 487–498.
Eastwood, S., Derish, P., Leash, E., & Ordway, S. (1996). Ethical issues in biomedical research: Perceptions and practices of postdoctoral research fellows responding to a survey. Science and Engineering Ethics, 2, 89–114.
Committee on Assessing Integrity in Research Environments (2002). Integrity in scientific research: Creating an environment that promotes responsible conduct. Washington, D.C.: Board on Health Sciences Policy and Division of Earth and Life Studies, Institute of Medicine and National Research Council of the National Academies, National Academies Press.
Hull, R., Wurm-Schaar, M., James-Valutis, M., & Triggle, R. & D. (1994). The Effect of a Research Ethics Course on Graduate Students’ Moral Reasoning. National Academy of Sciences, Washington, D.C. July 5–6, 1994. [available online at:http://www.richard-t-hull.com/publications/effect_research_ethics.pdf] Accessed 5/11/07.
Bebeau, M. J., Pimple, K. D., Muskavitch, K. M. T., & Smith, D. H. (1995). Moral reasoning in scientific research: A tool for teaching and assessment. Bloomington, IN.: Indiana University.
Heitman, E., Salis, P. J., & Bulger, R. E. (2000). Teaching ethics in biomedical science: Effects on moral reasoning skills. In Steneck, N. H. & Scheetz, M. D. (Eds.). Investigating research integrity. Proceedings of the First ORI Research Conference on Research Integrity. pp. 195–202. [available online at: http://www.ori.hhs.gov/documents/proceedings_rri.pdf] Accessed 5/11/07.
Kalichman, M. W., & Plemmons, D. K. (2007) Reported goals for responsible conduct of research courses. Academic Medicine in press.
Plemmons, D., Brody, S. A., & Kalichman, M. (2006). Student perceptions of the effectiveness of education in the responsible conduct of research. Science and Engineering Ethics, 12, 571–582.
Rest, J., Narvaez, D., Bebeau, M. J., & Thoma, S. J. (1999). Postconventional moral thinking: A Neo-Kohlbergian approach. Florence, KY.: Lawrence Erlbaum Associates.
De Vries, R., Anderson, M. S., & Martinson, B. C. (2006). Normal misbehavior: Scientists talk about the ethics of research. Journal of Empirical Research on Human Research Ethics, 1(1), 43–50.
Author information
Authors and Affiliations
Corresponding author
Appendix 1. Research Ethics Survey
Appendix 1. Research Ethics Survey
Note that three different versions (A, B, and C) of the survey were identical except for item number 22, which is one of three case scenarios. All three scenarios are included below.
-
1.
In what year did you take, or will you have taken, the UCSD School of Medicine summer course in research ethics?
2003 2002 2001 2000
-
2.
Have you taken any other course or received training in research ethics? Yes No
-
If
yes, how many years ago did you take the course or receive training? _____
-
3.
How many years of research experience do you have?
0 1 2 3 or more
-
4.
Who owns the data collected by a researcher at UCSD?
-
___
(a) the person who collected the data
-
___
(b) the researcher who heads the research group
-
___
(c) UCSD
-
___
(d) the agency that funded the research
-
___
(e) don’t know
-
___
-
5.
For federally funded research, raw data and other research records must be kept [please choose the best answer]:
-
___
(a) until the paper is submitted
-
___
(b) until the paper is accepted for publication
-
___
(c) until 3 years after the paper is published
-
___
(d) until the grant is finished
-
___
(e) until 3 years after the last expenditure report
-
___
(f) indefinitely
-
___
-
6.
Which of the following documents best summarizes the ethical principles on which human subjects research is judged in this country?
-
___
(a) Nuremberg Code
-
___
(b) Belmont Report
-
___
(c) Declaration of Helsinki
-
___
(d) none of the above
-
___
-
7.
Which of the following are primary ethical principles by which human subjects research in this country is judged? [check all that apply]
-
___
(a) community need
-
___
(b) justice
-
___
(c) beneficence
-
___
(d) quality of science
-
___
(e) respect for persons
-
___
-
8.
Which of the following committees has primary responsibility for reviewing research involving human subjects?
-
___
(a) CCC
-
___
(b) COI
-
___
(c) IACUC
-
___
(d) IRB
-
___
-
9.
Which of the following item(s) is/are not sufficient alone for authorship, according to guidelines of the International Committee of Medical Journal Editors? [check all that apply]
-
___
(a) acquisition of funding
-
___
(b) collection of data
-
___
(c) general supervision of the research group
-
___
(d) final approval of the version to be published
-
___
-
10.
According to guidelines of the International Committee of Medical Journal Editors, if a potential author has drafted an article or revised it critically for important intellectual content, and has given his or her final approval of the version to be published, which of the following substantial contributions is sufficient to warrant authorship? [check all that apply]
-
___
(a) conception and design
-
___
(b) acquisition of data
-
___
(c) analysis and interpretation of data
-
___
(d) acquisition of funding
-
___
-
11.
Which of the following best characterizes the criteria for authorship in the biomedical sciences?
-
___
(a) standards of authorship vary widely
-
___
(b) standards of authorship are mandated by federal regulation
-
___
(c) standards of authorship are covered by guidelines widely accepted by practicing scientists
-
___
(d) eligibility for authorship depends more on performing the work than on generating ideas
-
___
(e) eligibility for authorship depends more on generating ideas than on performing the work
-
___
-
12.
Which of the following is/are included under the definition of Research Misconduct in the UCSD Integrity of Research Policy? [check all that apply]
-
___
(a) fabrication
-
___
(b) falsification
-
___
(c) plagiarism
-
___
(d) financial conflicts of interest
-
___
(e) other practices that seriously deviate from those that are commonly accepted within the scientific community
-
___
For Questions 13–19, please indicate your reaction to the statement using the scale below
-
1=strongly disagree
-
2=disagree
-
3=neither agree nor disagree
-
4=agree
-
5=strongly agree
-
13.
Sloppy record keeping in research should be considered an example of research misconduct. ___
-
14.
When publishing data that you had previously published it is not necessary to cite the previous publication.___
-
15.
Any research use of animal subjects is acceptable as long as the experiments do not cause unnecessary pain or suffering.___
-
16.
If you were concerned that someone senior to you was conducting experiments on cats that might as easily be conducted with frogs, then you would be willing to raise this issue with them.___
-
17.
Someone who has witnessed misconduct has an obligation to act.___
-
18.
If you witnessed misconduct, then you would be willing to report that misconduct.___
-
19.
Formal training in the responsible conduct of research should be required of all researchers. ___
20. During the past 3 months, how many conversations about research ethics have you had with other medical students outside of class?
0 1 2 3 or more
21. During the past 3 months, how many conversations about research ethics have you had with faculty or other researchers outside of class?
0 1 2 3 or more
Case scenario A
22. Alana is a medical student researcher in the laboratory of Prof. Hayes. Prof. Hayes has received a manuscript for review for possible publication in a biomedical journal and asks Alana to review the manuscript. Alana knows that the review process is intended to be confidential, so she asks if the journal editor has been notified of this request. Prof. Hayes says that this is not necessary. Alana asks for your advice.
-
(a)
Is Professor Hayes’ answer (that notification is not necessary) ethical?
-
Yes
No
-
(b)
Why or why not?
Case scenario B
22. Xiao is a medical student working on his first research project. While working late in the laboratory, he notices that Claudia, a senior postdoc, left her lab notebook out and open in a common area of the lab. As Xiao picks up the notebook to return it to her desk, he sees that the entry dated for today shows measurements that would have been obtained from a piece of lab equipment for which Xiao has been the sole user for the last 2 days. It would not be possible that Claudia had made those measurements at this time. Xiao is considering that he should say or do nothing about his observation, but he asks for your advice.
-
(a)
Is Xiao’s decision (to say or do nothing) ethical?
-
Yes
No
-
(b)
Why or why not?
Case scenario C
22. Eduardo is a medical student assigned to an interview study of elderly subjects with Alzheimer’s disease. Although these subjects had the capacity to consent at the time of the onset of the study, some of them are now suffering from severe dementia. In Eduardo’s most recent interview of one of these subjects (Molly), he found that she repeatedly punctuated her answers with the statement: “Don’t ask me any more questions.” However, Molly continued to answer Eduardo’s questions, despite a clearly diminished capacity to understand what was being asked. On the assumption that Molly had chosen to participate at a time when she still had the ability to offer informed consent, Eduardo decided to complete the interview. However, he is now asking you for your advice about keeping Molly in the study.
-
(a)
Was Eduardo’s decision (to keep Molly in the study) ethical?
-
Yes
No
-
(b)
Why or why not?
Rights and permissions
About this article
Cite this article
Powell, S.T., Allison, M.A. & Kalichman, M.W. Effectiveness of a responsible conduct of research course: a preliminary study. SCI ENG ETHICS 13, 249–264 (2007). https://doi.org/10.1007/s11948-007-9012-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11948-007-9012-y