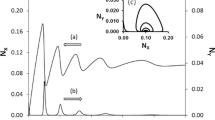
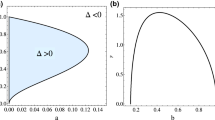
Abstract
Nonlinear oscillatory systems, playing a major role in biology, do not exhibit harmonic oscillations. Therefore, one might assume that the average value of any of their oscillating variables is unequal to the steady-state value. For a number of mathematical models of calcium oscillations (e.g. the Somogyi–Stucki model and several models developed by Goldbeter and co-workers), the average value of the cytosolic calcium concentration (not, however, of the concentration in the intracellular store) does equal its value at the corresponding unstable steady state at the same parameter values. The average value for parameter values in the unstable region is even equal to the level at the stable steady state for other parameter values, which allow stability. This holds for all parameters except those involved in the net flux across the cell membrane. We compare these properties with a similar property of the Higgins–Selkov model of glycolytic oscillations and two-dimensional Lotka–Volterra equations. Here, we show that this equality property is critically dependent on the following conditions: There must exist a net flux across the model boundaries that is linearly dependent on the concentration variable for which the equality property holds plus an additive constant, while being independent of all others. A number of models satisfy these conditions or can be transformed such that they do so. We discuss our results in view of the question which advantages oscillations may have in biology. For example, the implications of the findings for the decoding of calcium oscillations are outlined. Moreover, we elucidate interrelations with metabolic control analysis.







Similar content being viewed by others
References
Berridge MJ (1995) Capacitative calcium entry. Biochem J 312:1–11
Berridge MJ, Bootman MD, Lipp P (1998) Calcium—a life and death signal. Nature 395:645–648
Bolotina VM, Csutora P (2005) CIF and other mysteries of the store-operated Ca2+-entry pathway. Trends Biochem Sci 30:378–387
Borghans JAM, Dupont G, Goldbeter A (1997) Complex intracellular calcium oscillations. A theoretical exploration of possible mechanisms. Biophys Chem 66:25–41
Camello P, Gardner J, Petersen OH, Tepikin AV (1996) Calcium dependence of calcium extrusion and calcium uptake in mouse pancreatic acinar cells. J Physiol 490:585–593
Carafoli E (1991) Calcium pump of the plasma membrane. Physiol Rev 71:129–153
De Koninck P, Schulman H (1998) Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 279:227–230
Dolmetsch RE, Xu K, Lewis RS (1998) Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392:933–936
Draber P, Draberova L (2005) Lifting the fog in store-operated Ca2+ entry. Trends Immunol 26:621–624
Dupont G, Goldbeter A (1992) Protein phosphorylation driven by intracellular calcium oscillations: a kinetic analysis. Biophys Chem 42:257–270
Dupont G, Goldbeter A (1993) One-pool model for Ca2+ oscillations involving Ca2+ and inositol 1,4,5-triphosphate as co-agonists for Ca2+ release. Cell Calcium 14:311–322
Dupont G, Goldbeter A (1998) CaM kinase II as frequency decoder of Ca2+ oscillations. Bioessays 20:607–610
Dupont G, Swillens S, Clair C, Tordjmann T, Combettes L (2000) Hierarchical organization of calcium signals in hepatocytes: from experiments to models. Biochim Biophys Acta 1498:134–152
Dupont G, Houart G, De Koninck P (2003) Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations: a simple model. Cell Calcium 34:485–497
Falcke M (2004) Reading the patterns in living cells—the physics of Ca2+ signalling. Adv Phys 53:255–440
Fall CP, Keizer JE (2001) Mitochondrial modulation of intracellular Ca2+ signaling. J Theor Biol 210(2):151–165
Fell D (1997) Understanding the control of metabolism. Portland Press, London
Gall D, Baus E, Dupont G (2000) Activation of the liver glycogen phosphorylase by Ca2+ oscillations: a theoretical study. J Theor Biol 207:445–454
Goldbeter A (1996) Biochemical oscillations and cellular rhythms. Cambridge University Press, Cambridge
Goldbeter A, Dupont G, Berridge MJ (1990) Minimal model for signal-induced calcium oscillations and for their frequency encoding through protein phosphorylation. Proc Natl Acad Sci USA 87:1461–1465
Hauser MJB, Kummer U, Larsen AZ, Olsen LF (2001) Oscillatory dynamics protect enzymes and possibly cells against toxic substances. Faraday Discuss 120:215–227
Heinrich R., Rapoport T (1974) A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur J Biochem 42:89–95
Heinrich R, Schuster S (1996) The regulation of cellular systems. Chapman and Hall, New York
Higgins J (1964) A chemical mechanism for oscillation of glycolytic intermediates in yeast cells. Proc Natl Acad Sci USA 51:989–994
Hofbauer J, Sigmund K (1998) Evolutionary games and population dynamics. Cambridge University Press, Cambridge
Höfer T (1999) Model of intercellular calcium oscillations in hepatocytes: synchronization of heterogeneous cells. Biophys J 77:1244–1256
Houart G, Dupont G, Goldbeter A (1999) Bursting, chaos and birhythmicity originating from self-modulation of the inositol 1,4,5-trisphosphate signal in a model for intracellular Ca2+ oscillations. Bull Math Biol 61:507–530
Kholodenko BN, Schuster S, Garcia J, Westerhoff HV, Cascante M (1998) Control analysis of metabolic systems involving quasi-equilibrium reactions. Biochim Biophys Acta 1379:337–352
Kowalewski JM, Uhlen P, Kitano H, Brismar H (2006) Modeling the impact of store-operated Ca2+ entry on intracellular Ca2+ oscillations. Math Biosci 204:232–249
Kummer U, Olsen LF, Dixon CJ, Green AK, Bornberg-Bauer E, Baier G (2000) Switching from simple to complex oscillations in calcium signaling. Biophys J 79:1188–1195
Li Y, Stojilkovic S, Keizer J, Rinzel J (1997) Sensing and refilling calcium stores in an excitable cell. Biophys J 72:1080–1091
Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr., Meyer T (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15:1235–1241
Madsen MF, Dano S, Sorensen PG (2005) On the mechanisms of glycolytic oscillations in yeast. FEBS J 272:2648–2660
Marhl M, Grubelnik V (2007) Role of cascades in converting oscillatory signals into stationary step-like responses. Biosystems 87:58–67
Marhl M, Schuster S, Brumen M, Heinrich R (1997) Modelling the interrelations between calcium oscillations and ER membrane potential oscillations. Biophys Chem 63:221–239
Marhl M, Schuster S, Brumen M (1998a) Mitochondria as an important factor in the maintenance of constant amplitudes of cytosolic calcium oscillations. Biophys Chem 71:125–132
Marhl M, Schuster S, Brumen M, Heinrich R (1998b) Modelling oscillations of calcium and endoplasmic reticulum transmembrane potential. Role of the signalling and buffering proteins and of the size of the Ca2+ sequestering ER subcompartments. Bioelectrochem Bioenerg 46:79–90
Marhl M, Haberichter T, Brumen M, Heinrich R (2000). Complex calcium oscillations and the role of mitochondria and cytosolic proteins. BioSystems 57:75–86
Marhl M, Perc M, Schuster S (2006) A minimal model for decoding of time-limited Ca2+ oscillations. Biophys Chem 120:161–167
Murray JD (2002) Mathematical biology, vol I. Springer, Berlin
Olsen LF, Hauser MJB, Kummer U (2003) Mechanism of protection of peroxidase activity by oscillatory dynamics. Eur J Biochem (FEBS) 270:2796–2804
Palsson BO (2006) Systems biology. Properties of reconstructed networks. Cambridge University Press, Cambridge
Papin JA, Stelling J, Price ND, Klamt S, Schuster S, Palsson BO (2004) Comparison of network-based pathway analysis methods. Trends Biotechnol 22:400–405
Parekh AB, Putney JW Jr (2005) Store-operated calcium channels. Physiol Rev 85:757–810
Putney JW Jr (2005) Capacitative calcium entry: sensing the calcium stores. J Cell Biol 169:381–382
Randriamampita C, Tsien RY (1993) Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature 364:809–814
Reder C (1988) Metabolic control theory: a structural approach. J theor Biol 135:175–201
Reijenga KA, Westerhoff HV, Kholodenko BN, Snoep JL (2002) Control analysis for autonomously oscillating biochemical networks. Biophys J 82:99–108
Ritter AB, Douglas JM (1970) Frequency response of nonlinear systems. IEC Fundam 9:21–28
Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S et al (2005) STIM1 an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169:435–445
Rozi A, Jia Y (2003) A theoretical study of effects of cytosolic Ca2+ oscillations on activation of glycogen phosphorylase. Biophys Chem 106:193–202
Rudin W (1987) Real and complex analysis. McGraw-Hill, New York
Schuster S, Marhl M (2001) Bifurcation analysis of calcium oscillations: time-scale separation, canards, and frequency lowering. J Biol Syst 9:291–314
Schuster S, Schuster R (1992) Decomposition of biochemical reaction systems according to flux control insusceptibility. J Chim Phys 89:1887–1910
Schuster P, Sigmund K, Hofbauer J, Wolff R (1981) Selfregulation of behavior in animal societies. I. Symmetric contests. Biol Cybern 40:1–8
Schuster S, Marhl M, Höfer T (2002) Modelling of simple and complex calcium oscillations. From single-cell responses to intercellular signalling. Eur J Biochem 69:1333–1355
Schuster S, Knoke B, Marhl M (2005) Differential regulation of proteins by bursting calcium oscillations—a theoretical study. BioSystems 81:49–63
Selkov EE (1968) Self-oscillations in glycolysis. 1. A simple kinetic model. Eur J Biochem 4:79–86
Somogyi R, Stucki JW (1991) Hormone-induced calcium oscillations in liver cells can be explained by a simple one pool model. J Biol Chem 266:11068–11077
Stucki JW, Urbanczik R (2005) Entropy production of the Willamowski-Rössler oscillator. Z Naturforsch 60a:599–607
Teusink B, Westerhoff HV (2000) ‘Slave’ metabolites and enzymes. A rapid way of delineating metabolic control. Eur J Biochem 267:1889–1893
Thomas D, Hanley MR (1995) Evaluation of calcium influx factors from stimulated Jurkat T-lymphocytes by microinjection into Xenopus oocytes. J Biol Chem 270:6429–6432
Thompson JMT, Stewart HB (2002) Nonlinear dynamics and chaos. Wiley, Chichester, UK, p 437
Walter W (2000) Gewöhnliche Differentialgleichungen. Springer, Berlin
Wiesner TF, Berk BC, Nerem RM (1996) A mathematical model of cytosolic calcium dynamics in human umbilical vein endothelial cells. Am J Physiol Cell Physiol 270:C1556–C1569
Willamowski KD, Rössler OE (1980) Irregular oscillations in a realistic abstract quadratic mass-action system. Z Naturforsch A 35:317–318
Wolf J, Heinrich R (2000) Effect of cellular interaction on glycolytic oscillations in yeast: a theoretical investigation. Biochem J 345:321–334
Woods NM, Cuthbertson KSR, Cobbold PH (1986) Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature 319:600–602
Acknowledgments
Travel grants from the Slovenian and German Ministries of Research and Education (grant nos. BI-DE/03-04-003 and SVN 02/013, respectively) and from the E.U. within the SOCRATES program for mutual working and teaching visits are gratefully acknowledged. We thank Gunter Neumann for drawing our attention to mathematical properties of Lotka–Volterra systems and Reinhart Heinrich, Thomas Höfer, Ursula Kummer, Jörg Stucki and Jana Wolf for stimulating discussions. We are grateful to Ina Weiß for valuable assistance in the literature search.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to the memory of the late Reinhart Heinrich, who was the academic teacher of S.S. and, to a great extent, also of M.M.
Rights and permissions
About this article
Cite this article
Knoke, B., Marhl, M., Perc, M. et al. Equality of average and steady-state levels in some nonlinear models of biological oscillations. Theory Biosci. 127, 1–14 (2008). https://doi.org/10.1007/s12064-007-0018-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12064-007-0018-4