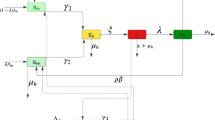
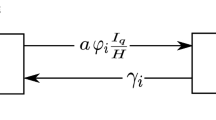Abstract
Malaria, a lethal protozoan disease transmitted through the bites of female Anopheles mosquitoes infected with Plasmodium parasites, remains a significant global health concern. This study introduces a compartmental mathematical model to explore the impact of insecticide use and malaria treatment based on awareness initiatives. The model incorporates the influence of media-based awareness on the effectiveness of insecticide utilization for malaria control. Key mathematical properties, such as positivity, boundedness of solutions, feasibility, and stability of equilibria, are systematically investigated. Our analysis demonstrates that all solutions to the system are positive and bounded within a specified set of initial conditions, establishing the mathematical soundness and epidemiological relevance of the model. The basic reproduction number \(R_0\) is determined through the next-generation matrix method. Stability analysis reveals that the disease-free equilibrium is globally asymptotically stable when \(R_0\) is less than one, while it becomes unstable if \(R_0\) exceeds one. Global stability of the endemic equilibrium is established using an appropriate quadratic Lyapunov function in cases where \(R_0\) surpasses one. We identify the most sensitive parameters of the model through normalized forward sensitivity indices. In addition, numerical simulations employing the Runge–Kutta method in Python software further validate our findings. Both analytical and numerical results collectively suggest that the integration of awareness-based insecticide usage with malaria treatment holds the potential for malaria elimination. This comprehensive approach not only contributes to the mathematical rigor of the model but also underscores its practical implications for effective malaria control strategies.











Similar content being viewed by others
Change history
30 July 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12190-024-02195-0
References
Organization, W.H., et al.: Malaria vaccine: Who position paper-march 2022. Wkly Epidemiol. Rec. 97(9), 61–80 (2022)
Cox, F.E.: History of the discovery of the malaria parasites and their vectors. Parasites Vectors 3, 1–9 (2010)
Sabbatani, S., Fiorino, S., Manfredi, R.: The emerging of the fifth malaria parasite (Plasmodium knowlesi): A public health concern? Braz. J. Infect. Diseases 14, 299–309 (2010)
Kendie, F.A., Hailegebriel W/kiros, T., Nibret Semegn, E., Ferede, M.W.: Prevalence of malaria among adults in Ethiopia: a systematic review and meta-analysis. J. Trop. Med. 2021, 1–9 (2021)
Kojom, L.P., Singh, V.: Prevalence of Plasmodium falciparum field isolates with deletions in histidine-rich protein 2 and 3 genes in context with Sub-Saharan Africa and India: a systematic review and meta-analysis. Malar. J. 19, 1–14 (2020)
Wang, C., Thakuri, B., Roy, A.K., Mondal, N., Qi, Y., Chakraborty, A.: Changes in the associations between malaria incidence and climatic factors across malaria endemic countries in Africa and Asia-Pacific region. J. Environ. Manage. 331, 117264 (2023)
Keno, T.D., Makinde, O.D., Obsu, L.L.: Impact of temperature variability on sirs malaria model. J. Biol. Syst. 29(03), 773–798 (2021)
Tchoumi, S.Y., Chukwu, C., Diagne, M.L., Rwezaura, H., Juga, M., Tchuenche, J.M.: Optimal control of a two-group malaria transmission model with vaccination. Netw. Model. Anal. Health Inform. Bioinform. 12(1), 7 (2022)
Anjorin, S., Okolie, E., Yaya, S.: Malaria profile and socioeconomic predictors among under-five children: an analysis of 11 sub-Aaharan African countries. Malar. J. 22(1), 55 (2023)
Kaindoa, E.W., Mmbando, A.S., Shirima, R., Hape, E.E., Okumu, F.O.: Insecticide-treated eave ribbons for malaria vector control in low-income communities. Malar. J. 20, 1–12 (2021)
Sougoufara, S., Ottih, E.C., Tripet, F.: The need for new vector control approaches targeting outdoor biting anopheline malaria vector communities. Parasites Vectors 13, 1–15 (2020)
Fischer, P.R.: Emerging options for malaria prevention. Infect. Disease Alert 41(1) (2021)
Bazán, M.J.A., Mora, C.F.: Compliance with preventive measures against malaria of personnel treated in the Centre of International Vaccination of the Minister of Defence (Spain). Rev. Esp. Quimioter. 33(1), 11 (2020)
Asale, A., Abro, Z., Enchalew, B., Teshager, A., Belay, A., Kassie, M., Mutero, C.M.: Community knowledge, perceptions, and practices regarding malaria and its control in Jabi Tehnan District, Amhara Region, Northwest Ethiopia. Malar. J. 20, 1–13 (2021)
Mekuriaw, W., Yewhalaw, D., Woyessa, A., Bashaye, S., Massebo, F.: Distribution and trends of insecticide resistance in malaria vectors in Ethiopia (1986–2017): a review. Ethiop. J. Public Health Nutr. (EJPHN) 3, 51–61 (2019)
Collins, O., Duffy, K.: A mathematical model for the dynamics and control of malaria in Nigeria. Infect. Disease Model. 7(4), 728–741 (2022)
Egwu, C.O., Aloke, C., Chukwu, J., Nwankwo, J.C., Irem, C., Nwagu, K.E., Nwite, F., Agwu, A.O., Alum, E., Offor, C.E., et al.: Assessment of the antimalarial treatment failure in Ebonyi State, Southeast Nigeria. J. Xenobiot. 13(1), 16–26 (2023)
Zuber, J.A., Takala-Harrison, S.: Multidrug-resistant malaria and the impact of mass drug administration. Infect. Drug Resist. 299–306 (2018)
Ibrahim, M.M., Kamran, M.A., Naeem Mannan, M.M., Kim, S., Jung, I.H.: Impact of awareness to control malaria disease: a mathematical modeling approach. Complexity 2020, 1–13 (2020)
Doda, Z., Solomon, T., Loha, E., Gari, T., Lindtjørn, B.: A qualitative study of use of long-lasting insecticidal nets (llins) for intended and unintended purposes in Adami Tullu, East Shewa zone, Ethiopia. Malar. J. 17, 1–14 (2018)
Basir, F.A., Banerjee, A., Ray, S.: Exploring the effects of awareness and time delay in controlling malaria disease propagation. Int. J. Nonlinear Sci. Numer. Simul. 22(6), 665–683 (2021)
Kasteng, F., Murray, J., Cousens, S., Sarrassat, S., Steel, J., Meda, N., Ouedraogo, M., Head, R., Borghi, J.: Cost-effectiveness and economies of scale of a mass radio campaign to promote household life-saving practices in Burkina Faso. BMJ Glob. Health 3(4), 000809 (2018)
Mutabingwa, T.K.: Artemisinin-based combination therapies (acts): best hope for malaria treatment but inaccessible to the needy! Acta Trop. 95(3), 305–315 (2005)
Abanyie, F., Acharya, S.D., Leavy, I., Bowe, M., Tan, K.R.: Safety and effectiveness of intravenous Artesunate for treatment of severe malaria in the united states-April 2019 through December 2020. Clin. Infect. Dis. 73(11), 1965–1972 (2021)
Bloland, P.B.: Drug resistance in malaria (2001)
Tumwiine, J., Mugisha, J., Luboobi, L.S.: A mathematical model for the dynamics of malaria in a human host and mosquito vector with temporary immunity. Appl. Math. Comput. 189(2), 1953–1965 (2007)
Abioye, A.I., Ibrahim, M.O., Peter, O.J., Ogunseye, H.A.: Optimal control on a mathematical model of malaria. Sci. Bull. Series A: Appl. Math. Phys. 82(3), 177–190 (2020)
Romero-Leiton, J.P., Ibargüen-Mondragón, E.: Stability analysis and optimal control intervention strategies of a malaria mathematical model. Appl. Sci. 21 (2019)
Bacaër, N., Bacaër, N.: Ross and malaria (1911). A short history of mathematical population dynamics, 65–69 (2011)
Labadin, J., Kon, C., Juan, S.: Deterministic malaria transmission model with acquired immunity. In: Proceedings of the World Congress on Engineering and Computer Science, vol. 2, pp. 20–22 (2009)
Macdonald. G, M.G.: The epidemiology and control of malaria (1957)
Ngwa, G.A., Shu, W.S.: A mathematical model for endemic malaria with variable human and mosquito populations. Math. Comput. Model. 32(7–8), 747–763 (2000)
Anderson, R., May, R.: Infectious diseases of humans: dynamics and control (1991)
Chitnis, N., Hyman, J.M., Cushing, J.M.: Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull. Math. Biol. 70, 1272–1296 (2008)
Aldila, D., Angelina, M.: Optimal control problem and backward bifurcation on malaria transmission with vector bias. Heliyon 7(4) (2021)
Herdicho, F.F., Chukwu, W., Tasman, H., et al.: An optimal control of malaria transmission model with mosquito seasonal factor. Results Phys. 25, 104238 (2021)
Collins, O.C., Duffy, K.J.: Mathematical analyses on the effects of control measures for a waterborne disease model with socioeconomic conditions. J. Comput. Biol. 28(1), 19–32 (2021)
Chiyaka, C., Garira, W., Dube, S.: Transmission model of endemic human malaria in a partially immune population. Math. Comput. Model. 46(5–6), 806–822 (2007)
Yang, H.M.: Malaria transmission model for different levels of acquired immunity and temperature-dependent parameters (vector). Rev. Saude Publica 34, 223–231 (2000)
Dietz, K., Molineaux, L., Thomas, A.: A malaria model tested in the African Savannah. Bull. World Health Organ. 50(3–4), 347 (1974)
Ngonghala, C.N., Wairimu, J., Adamski, J., Desai, H.: Impact of adaptive mosquito behavior and insecticide-treated nets on malaria prevalence. J. Biol. Syst. 28(02), 515–542 (2020)
Srivastav, A.K., Ghosh, M.: Modeling the transmission dynamics of malaria with saturated treatment: a case study of India. J. Appl. Math. Comput. 67(1), 519–540 (2021)
Agusto, F.B., Del Valle, S.Y., Blayneh, K.W., Ngonghala, C.N., Goncalves, M.J., Li, N., Zhao, R., Gong, H.: The impact of bed-net use on malaria prevalence. J. Theor. Biol. 320, 58–65 (2013)
Al Basir, F., Abraha, T.: Mathematical modelling and optimal control of malaria using awareness-based interventions. Mathematics 11(7), 1687 (2023)
Al Basir, F., Blyuss, K.B., Ray, S.: Modelling the effects of awareness-based interventions to control the mosaic disease of Jatropha curcas. Ecol. Complex. 36, 92–100 (2018)
Heesterbeek, J., Dietz, K.: The concept of RO in epidemic theory. Stat. Neerl. 50(1), 89–110 (1996)
Heffernan, J.M., Smith, R.J., Wahl, L.M.: Perspectives on the basic reproductive ratio. J. R. Soc. Interface 2(4), 281–293 (2005)
Driessche, P., Watmough, J.: Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 180(1–2), 29–48 (2002)
Dolai, S., Roy, A.K., Roy, P.K.: Mathematical study on human cells interaction dynamics for hiv-tb co-infection. In: Mathematical Modelling, Optimization, Analytic and Numerical Solutions, pp. 351–366. Springer (2020)
Roy, P.K., Roy, A.K., Khailov, E.N., Al Basir, F., Grigorieva, E.V.: A model of the optimal immunotherapy of psoriasis by introducing il-10 and il-22 inhibitors. J. Biol. Syst. 28(03), 609–639 (2020)
Roy, A.K., Al Basir, F., Roy, P.K.: A vivid cytokines interaction model on psoriasis with the effect of impulse biologic (tnf- \(\alpha \) inhibitor) therapy. J. Theor. Biol. 474, 63–77 (2019)
Ojo, M.M., Doungmo Goufo, E.F.: Assessing the impact of control interventions and awareness on malaria: a mathematical modeling approach. Commun. Math. Biol. Neurosci. (2021)
Kanyi, E., Afolabi, A.S., Onyango, N.O.: Mathematical modeling and analysis of transmission dynamics and control of schistosomiasis. J. Appl. Math. 2021, 1–20 (2021)
Castillo-Chavez, C., Song, B.: Dynamical models of tuberculosis and their applications. Math. Biosci. Eng. 1(2), 361–404 (2004)
Cangiotti, N., Capolli, M., Sensi, M., Sottile, S.: A survey on lyapunov functions for epidemic compartmental models. Bollettino dell’Unione Matematica Italiana 2024, 17(2), 241–257
La Salle, J.P.: An invariance principle in the theory of stability. Technical report (1966)
Samsuzzoha, M., Singh, M., Lucy, D.: Uncertainty and sensitivity analysis of the basic reproduction number of a vaccinated epidemic model of influenza. Appl. Math. Model. 37(3), 903–915 (2013)
Strogatz, S.H.: Nonlinear dynamics and chaos: Lab demonstrations (1994)
Hirsch, M.W., Smale, S., Devaney, R.L.: Differential Equations, Dynamical Systems, and an Introduction to Chaos. Academic Press, London (2012)
Siriprapaiwan, S., Moore, E.J., Koonprasert, S.: Generalized reproduction numbers, sensitivity analysis and critical immunity levels of an seqijr disease model with immunization and varying total population size. Math. Comput. Simul. 146, 70–89 (2018)
Wang, X., Chen, Y., Liu, S.: Dynamics of an age-structured host-vector model for malaria transmission. Math. Methods Appl. Sci. 41(5), 1966–1987 (2018)
Keno, T.D., Obsu, L.L., Makinde, O.D.: Modeling and optimal control analysis of malaria epidemic in the presence of temperature variability. Asian-Eur. J. Math. 15(01), 2250005 (2022)
Acknowledgements
The authors would like to thank Adama Science and Technology University (ASTU) for offering resources and steadfast support during this research article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this research article declare that there are no Conflict of interest in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A: Routh–Hurwitz stability criteria for the characteristic polynomial (26)
Appendix A: Routh–Hurwitz stability criteria for the characteristic polynomial (26)
Based on the relationship between the coefficients and the trace/determinant, we can write the expression for the characteristic polynomial coefficients as follows:
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haringo, A.T., Obsu, L.L. & Bushu, F.K. A mathematical model of malaria transmission with media-awareness and treatment interventions. J. Appl. Math. Comput. 70, 4715–4753 (2024). https://doi.org/10.1007/s12190-024-02154-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12190-024-02154-9