Abstract
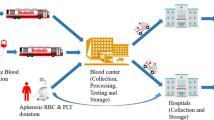
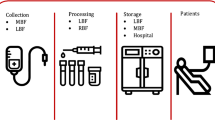
Motivated by a real issue in Iran blood transfusion organization and hemophilia community, this paper aims to design and plan an integrated plasma and plasma-derived medicines supply chain network to improve efficiency and timely access to high-quality services. This paper contributes to the existing literature by incorporating four factors, namely (1) quality, (2) efficiency, (3) type-differentiated demand, and (4) risk controlling into the model. To better manage the quality of plasma, we categorize fresh frozen plasma into two classes based on plasma freezing time interval. Moreover, since the number of available donors and centers accessibility affects the supply chain performance, the ideal candidate locations of plasma donation centers are evaluated through several pivotal criteria via a data envelopment analysis approach. We propose a mathematical model, based on a tactical-strategic decision in a real case. Furthermore, the robustness manages the inherent uncertainty in input data and controls the risk of probable disruption in the model, simultaneously. We consider a real case study according to hemophilia community issues in Tehran. The outcomes assist the practitioners in managing how plasma products should be manufactured to minimize the shortages and also control the robustness against uncertainty and disruption risks.









Similar content being viewed by others
References
Ala FA (2016) The history of hemophilia in Iran. Arch Iran Med 19(3):229–232
Anderson NL, Anderson NG (2002) The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics 1(11):845–867
Beliën J, Forcé H (2012) Supply chain management of blood products: a literature review. Eur J Oper Res 217(1):1–6
Ben-Tal A, Nemirovski A (1999) Robust solutions of uncertain linear programs. Oper Res Lett 25(1):1–3
Ben-Tal A, El-Ghaoui L, Nemirovski A (2009) Robust optimization. Princeton University Press, Princeton
Bill S (2019) Canadian health coalition. Policy 613:688–4973
Charnes A, Cooper WW, Rhodes E (1978) Measuring the efficiency of decision making units. Eur J Oper Res 2(6):429–444
Cheraghali AM (2012) Overview of blood transfusion system of Iran: 2002–2011. Iran J Public Health 41(8):89
Cheraghali AM, Abolghasemi H (2009) Plasma fractionation, a useful means to improve national transfusion system and blood safety: Iran experience. Haemophilia 15(2):487–493
Cheraghali AM, Aboofazeli R (2009) Economical impact of plasma fractionation project in Iran on affordability of plasma-derived medicines. Transfus Med 19(6):363–368
Cheraghi S, Hosseini-Motlagh SM (2018) Responsive and reliable injured-oriented blood supply chain for disaster relief: a real case study. Ann Oper Res. https://doi.org/10.1007/s10479-018-3050-5
Cohn EJ, Strong LE, Hughes W, Mulford DJ, Ashworth JN, Melin ME, Taylor HL (1946) Preparation and properties of serum and plasma proteins. IV. A system for the separation into fractions of the protein and lipoprotein components of biological tissues and fluids1a, b, c, d. J Am Chem Soc 68(3):459–475
Daskin MS, Dean LK (2006) Location of health care facilities. Oper Res Health Care. https://doi.org/10.1007/1-4020-8066-2_3
De Angelis V, Breda A (2013) Plasma-derived medicinal products self-sufficiency from national plasma: to what extent? Blood Transfus 11(Suppl 4):s132
Desborough M, Sandu R, Brunskill SJ, Doree C, Trivella M, Montedori A, Abraha I, Stanworth S (2015) Fresh frozen plasma for cardiovascular surgery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD007614.pub2
Ensafian H, Yaghoubi S (2017) Robust optimization model for integrated procurement, production and distribution in platelet supply chain. Transp Res Part E Logist Transp Rev 1(103):32–55
Ensafian H, Yaghoubi S, Yazdi MM (2017) Raising quality and safety of platelet transfusion services in a patient-based integrated supply chain under uncertainty. Comput Chem Eng 2(106):355–372
Fahimnia B, Jabbarzadeh A, Ghavamifar A, Bell M (2017) Supply chain design for efficient and effective blood supply in disasters. Int J Prod Econ 1(183):700–709
Farrugia A (2004) Plasma for fractionation: safety and quality issues. Haemophilia 10(4):334–340
Farrugia A, Cassar J (2012) Plasma-derived medicines: access and usage issues. Blood Transfus 10(3):273
Goss N, Curling J (2013) The economics of plasma fractionation. Prod Plasma Proteins Ther Use 21:449–460
Govindan K, Jafarian A, Khodaverdi R, Devika K (2014) Two-echelon multiple-vehicle location–routing problem with time windows for optimization of sustainable supply chain network of perishable food. Int J Prod Econ 152:9–28
Haeri A, Hosseini-Motlagh S-M, Ghatreh Samani MR, Rezaei M (2019) A mixed resilient-efficient approach toward blood supply chain network design. Int Trans Oper Res 27:1962–2001
Hosseini KM, Ghasemzadeh M (2016) Implementation of plasma fractionation in biological medicines production. Iran J Biotechnol 14(4):213
Hosseini-Motlagh S-M, Ghatreh Samani MR, Homaei S (2019a) Blood supply chain management: robust optimization, disruption risk, and blood group compatibility (a real-life case). J Ambient Intell Humanized Comput 11:1085–1104
Hosseini-Motlagh S-M, Ghatreh-Samani MR, Saadi FA (2019b) Strategic optimization of wheat supply chain network under uncertainty: a real case study. In: Operational research (Issue 0123456789). Springer, Berlin Heidelberg. https://doi.org/10.1007/s12351-019-00515-y
Jabbarzadeh A, Fahimnia B, Seuring S (2014) Dynamic supply chain network design for the supply of blood in disasters: a robust model with real world application. Transp Res Part E Logist Transp Rev 1(70):225–244
Karam O, Lacroix J, Robitaille N, Rimensberger PC, Tucci M (2013) Association between plasma transfusions and clinical outcome in critically ill children: a prospective observational study. Vox Sang 104(4):342–349
Khalilpourazari S, Khamseh AA (2017) Bi-objective emergency blood supply chain network design in earthquake considering earthquake magnitude: a comprehensive study with real world application. Ann Oper Res. https://doi.org/10.1007/2Fs10479-017-2588-y
Larimi NG, Yaghoubi S (2019) A robust mathematical model for platelet supply chain considering social announcements and blood extraction technologies. Comput Ind Eng 137:106014
Larimi NG, Yaghoubi S, Hosseini-Motlagh S-M (2019) Itemized platelet supply chain with lateral transshipment under uncertainty evaluating inappropriate output in laboratories. Socio-Econ Plan Sci 68:100697
Levis AA, Papageorgiou LG (2004) A hierarchical solution approach for multi-site capacity planning under uncertainty in the pharmaceutical industry. Comput Chem Eng 28(5):707–725
Morfini M, Coppola A, Franchini M, Di Minno G (2013) Clinical use of factor VIII and factor IX concentrates. Blood Transfus 11(Suppl 4):s55
Mousazadeh M, Torabi SA, Zahiri B (2015) A robust possibilistic programming approach for pharmaceutical supply chain network design. Comput Chem Eng 2(82):115–128
Nasrollahi M, Razmi J (2019) A mathematical model for designing an integrated pharmaceutical supply chain with maximum expected coverage under uncertainty. Oper Res Int J. https://doi.org/10.1007/s12351-019-00459-3
Osorio AF, Brailsford SC, Smith HK (2015) A structured review of quantitative models in the blood supply chain: a taxonomic framework for decision-making. Int J Prod Res 53(24):7191–7212
Papageorgiou LG, Rotstein GE, Shah N (2001) Strategic supply chain optimization for the pharmaceutical industries. Ind Eng Chem Res 40(1):275–286
Park Q, Kim MJ, Lee J, Shin S (2010) Plasma fractionation in Korea: working towards self-sufficiency. Korean J Hematol 45(1):3–5
Peltoniemi T, Ihalainen J (2019) Evaluating blockchain for the governance of the plasma derivatives supply chain: how distributed ledger technology can mitigate plasma supply chain risks. Blockchain in Healthcare Today
Pishvaee MS, Rabbani M, Torabi SA (2011) A robust optimization approach to closed-loop supply chain network design under uncertainty. Appl Math Model 35(2):637–649
Rahmani D (2018) Designing a robust and dynamic network for the emergency blood supply chain with the risk of disruptions. Ann Oper Res. https://doi.org/10.1007/s10479-018-2960-6
Ramezanian R, Behboodi Z (2017) Blood supply chain network design under uncertainties in supply and demand considering social aspects. Transp Res Part E Logist Transp Rev 1(104):69–82
Rautonen J (2007) Redesigning supply chain management together with the hospitals. Transfusion 47:197S–200S
Rytilä JS, Spens KM (2006) Using simulation to increase efficiency in blood supply chains. Manag Res News 29(12):801–819
Sadjadi SJ, Omrani H, Abdollahzadeh S, Alinaghian M, Mohammadi H (2011) A robust super-efficiency data envelopment analysis model for ranking of provincial gas companies in Iran. Expert Syst Appl 38(9):10875–10881
Salehi F, Mahootchi M, Husseini SM (2017) Developing a robust stochastic model for designing a blood supply chain network in a crisis: a possible earthquake in Tehran. Ann Oper Res. https://doi.org/10.1007/s10479-017-2533-0
Samani MR, Hosseini-Motlagh SM (2018) An enhanced procedure for managing blood supply chain under disruptions and uncertainties. Ann Oper Res. https://doi.org/10.1007/s10479-018-2873-4
Samani MR, Hosseini-Motlagh SM, Sheshkol MI, Shetab-Boushehri SN (2019) A bi-objective integrated model for the uncertain blood network design with raising products quality. Eur J Ind Eng 13(5):553–588
Shaverdi Marzieh, Yaghoubi Saeed, Ensafian Hamidreza (2020) A multi-objective robust possibilistic model for technology portfolio optimization considering social impact and different types of financing. Appl Soft Comput 86:105892
Shim YJ, Lee KS, Kim UH, Suh JK, Baik SY, Hyun SY (2014) Progress of in vitro factor VIII coagulant activity from 0 to 8 hours after reconstitution. Blood Res 49(4):265–269
Snyder LV, Daskin MS (2006) Stochastic p-robust location problems. IIE Trans 38(11):971–985
Sousa RT, Shah N, Papageorgiou LG (2005) Global supply chain network optimization for pharmaceuticals. In: Computer aided chemical engineering, vol 20. Elsevier, pp 1189–1194
Swärd-Nilsson AM, Persson PO, Johnson U, Lethagen S (2006) Factors influencing factor VIII activity in frozen plasma. Vox Sang 90(1):33–39
Tullis JL, Albumin I (1977) Background and use. JAMA 237:355–360
WHO (2015) https://www.who.int/news-room/fact-sheets/detail/blood-safety-and-availability
Yaghoubi S, Hosseini-Motlagh S-M, Cheraghi S, Larimi NG (2019) Designing a robust demand-differentiated platelet supply chain network under disruption and uncertainty. J Ambient Intell Humanized Comput. https://doi.org/10.1007/s12652-019-01501-0
Zahiri B, Pishvaee MS (2017) Blood supply chain network design considering blood group compatibility under uncertainty. Int J Prod Res 55(7):2013–2033
Zahiri B, Torabi SA, Mousazadeh M, Mansouri SA (2015) Blood collection management: methodology and application. Appl Math Model 39(23–24):7680–7696
Zahiri B, Jula P, Tavakkoli-Moghaddam R (2018a) Design of a pharmaceutical supply chain network under uncertainty considering perishability and substitutability of products. Inf Sci 1(423):257–283
Zahiri B, Torabi SA, Mohammadi M, Aghabegloo M (2018b) A multi-stage stochastic programming approach for blood supply chain planning. Comput Ind Eng 1(122):1–4
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
Here are the data parameters for the problem investigation under the case study of Tehran. Noteworthy, transportation costs are provided based on the distance between the facilities which are obtained by Google map.
See Tables 9, 10, 11, 12 and 13.
Appendix 2
2.1 Notation
As mentioned in the study, the paper tries to solve some issues in IBTO and Hemophilia Community. Noteworthy, all these centers work under the supervision of IBTO (Cheraghali 2012), and the authors formulated and modeled the problem based on IBTO viewpoints. The notations of the proposed model are as follows:
Sets | |
N | Set of main blood centers, \(n = 1, \ldots ,N\) |
h | Set of hospitals, \(h = 1, \ldots , H\) |
\(o\) | Set of plasma collection centers, \(o = 1, \ldots ,O\) |
\(i\) | Set of blood research and fractionation company, \(i = 1, \ldots ,I\) |
\(e\) | Set of distribution centers, \(e = 1, \ldots ,E\) |
\(j\) | Set of pharmacy demand points, \(j = 1, \ldots ,J\) |
\(d\) | Set of FFP units age, \(d = 1,..,D\) |
\(v\) | Set of types of FFP, \(v = 1, \ldots ,V\) |
\(p\) | Set of PDMPs age, \(p = 1, \ldots ,P\) |
\(l\) | Set of types of PDMPs, l \(= 1, \ldots ,L\) |
\(t\) | Set of time periods, \(t = 1, \ldots ,T\) |
Parameters | |
\(C_{o}^{f}\) | Establishment cost of locating plasma collection centers \(o\) |
\(CC_{h }\) | Cost of inventory level of FFP units in main blood center \(n\) |
\(HC_{h }\) | Cost of inventory level of FFP units in hospital \(h\) |
\(OC_{o }\) | Cost of inventory level of FFP units in PLS collection center \(o\) |
\(DC_{e }\) | Cost of inventory level of PDMPs in distribution center \(e\) |
\(LC_{j }\) | Cost of inventory level of PDMPs in specialty pharmacy \(j\) |
\(TC^{tr}_{n,o }\) | Unit cost of FFP units shipped from the main blood center \(n\) to PLS collection center \(o\) |
\(MC ^{tr}_{n,h }\) | Unit cost of FFP units shipped from the main blood center \(n\) to hospital \(h\) |
\(BC^{tr}_{o,i }\) | Unit cost of FFP units shipped from PLS collection center \(o\) to BRF \(i\) |
\(KC^{tr}_{i,e }\) | Unit cost of PDMPs shipped from BRF \(i\) to distribution center \(e\) |
\(GC^{tr}_{e,j }\) | Unit cost of PDMPs shipped from distribution center \(e\) to specialty pharmacy j |
\(AC^{sa}_{n }\) | Unit cost of the whole blood centrifuge in the main blood center \(n\) |
\(ZC^{pr}_{n }\) | Unit cost of FFP units provided in the main blood center \(n\) |
\(RC^{aph}_{o }\) | Unit cost of apheresis process in PLS collection center \(o\) |
\(UC^{pr}_{o }\) | Unit cost of FFP units provided in PLS collection center \(o\) |
\(C^{w}_{n }\) | Wastage cost of FFP units in the main blood center \(n\) |
\(CC^{w}_{h }\) | Wastage cost of FFP units in hospital \(h\) |
\(EC^{w}_{e }\) | Wastage cost of PDMPs in distribution center \(e\) |
\(JC^{w}_{j }\) | Wastage cost of PDMPs in specialty pharmacy \(j\) |
\(SC^{sh}_{h }\) | Shortage cost of FFP units in hospital \(h\) |
\(JC^{sh}_{j}\) | Shortage cost of PDMPs in specialty pharmacy \(j\) |
\(wl_{n}\) | Overall capacity of collecting FFP units in the main blood center \(n\) |
\(kl_{n}\) | Capacity of collecting FFP units in the main blood center \(n\) |
\(ss_{o}^{'}\) | Capacity of maintenance FFP units in PLS collection center \(o\) |
\(k_{i,l}\) | Supply capacity of PDMPs \(l\) in BRF \(i\) |
\(w_{e,l }\) | Capacity of storage PDMPs \(l\) in distribution center \(e\) |
\(\phi_{n}\) | Maximum rate of plasma extracted from whole blood in blood center \(n\) |
\(\zeta_{\text{o}}\) | Maximum rate of FFP units extracted from PLS in PLS collection center \(o\). |
\(qa_{n,t }\) | Maximum blood donation (available donor) in blood center \(n\) in period \(t\) |
\(eh_{o,t }\) | Maximum plasma donation in PLS collection center \(o\) in period \(t\) |
\(dd_{h,d,t }\) | Demand of FFP units with age \(d\) in hospitals \(h\) in period \(t\) |
\(db_{j,p,l,t }\) | Demand of PDMPs type \(l\) with age \(p\) in specialty pharmacy \(j\) in period t |
\(sf_{j,l,t}\) | Safety stock level of PDMPs \(l\) in specialty pharmacy \(j\) in period \(t\) |
\(z_{l }\) | The proportion of producing each PDMPs |
\(M\) | A very large number |
Binary variables | |
\(\varPsi_{o }\) | \(1\); if the PLS collection center located in candidate location \(o\); \(0\) otherwise |
\(\beta_{n,t }\) | 1; if the freezers has enough capacity to freeze PLS in 2–4 h in period \(t\); 0 otherwise |
\(\theta_{v,t }\) | 1; if FFP units produced with type \(v\) allocated to hospital in period \(t;\) 0 otherwise |
\(y_{j,l,t }\) | 1; if the specialty pharmacy is compelled to use safety stock in period \(t;\) 0 otherwise |
\({{\Omega }}_{n,t }\) | 1; if PLS is extracted at the main blood center n in period t; 0 otherwise |
Positive variables | |
\(x_{n,o,v,d,t }\) | Quantity of FFP units with age \(d\) and type \(v\) shipped from blood center \(n\) to PLS collection center \(o\) in period \(t\) |
\(q^{\prime\prime}_{{n,h,{\text{d}},v,t }}\) | Quantity of FFP units with age \(d\) and type \(v\) shipped from the main blood center \(n\) to hospital \(h\) in period \(t\) |
\(gq_{o,i,t}\) | Quantity of FFP units shipped from PLS collection center \(o\) to BRF company \(i\) in period \(t\) |
\(u_{i,e,l,t }\) | Quantity of PDMPs \(l\) shipped from BRF company \(i\) to distribution center \(e\) in period \(t\) |
\(v_{e,j,l,p,t }\) | Quantity of PDMPs \(l\) with age \(p\) shipped from distribution center \(e\) to specialty pharmacy \(j\) in period \(t\) |
\(Nv^{ }_{n,d,v,t}\) | Inventory level of FFP units with age \(d\) and with type \(v\) in blood center \(n\) in period \(t\) |
\(Hv _{h,v,d,t}\) | Inventory level of FFP units with age \(d\) and type \(v\) in hospital \(h\) in period \(t\) |
\(Pv _{{{\text{o}},{\text{t}}}}\) | Inventory level of FFP units in PLS collection center \(o\) in period \(t\) |
\(Ev_{l,p,e,t}\) | Inventory level of PDMPs \(l\) with age \(p\) in distribution center \(e\) in period \(t\) |
\(Jv_{j,l,p,t }\) | Inventory level of PDMPs \(l\) with age \(p\) in specialty pharmacy \(j\) in period \(t\) |
\(hs_{h,t }\) | Shortage level of FFP units in hospital \(h\) in period \(t\) |
\(js_{j,l,t }\) | Shortage level of PDMPs \(l\) in specialty pharmacy \(j\) in period \(t\) |
\(rw_{n,v,t }\) | Wastage level of FFP units with type \(v\) in blood center \(n\) in period \(t\) |
\(hw_{h,v,t }\) | Wastage level of FFP units with type \(v\) in hospital \(h\) in period \(t\) |
\(ew_{e,l,t }\) | Wastage level of PDMPs \(l\) in distribution center \(e\) period \(t\) |
\(jw_{j,l,t }\) | Wastage level of PDMPs \(l\) in specialty pharmacy \(j\) period \(t\) |
\(sq_{n,t }\) | Quantity of blood donation in blood center \(n\) in period \(t\) |
\(qq_{n,v,t }\) | Quantity of plasma extracted from whole blood in the main blood center \(n\) by considering type \(v\) in period \(t\) |
\(q_{o,t }\) | Quantity of plasmapheresis donation in PLS collection center o in period t |
\(q^{\prime}_{o,t }\) | Quantity of FFP units in PLS collection center \(o\) in period t |
2.2 Robust optimization
In this study, based on different types of patients in hospitals as moderate and severe type, and specialty pharmacies (patients require Albumin, IVIG, or Factor VIII), two types of demand including FFP units and PDMPs \((\widetilde{dd}_{h,\varphi ,t } , \widetilde{db}_{j,p,l,t } )\) are considered as unknown parameters. Note that the transportation cost parameters (\(\widetilde{MC}_{n,h }\), \(\widetilde{TC}_{n,o }\), \(\widetilde{BC}_{o,i }\), \(\widetilde{KC}_{i,e }\) and \(\widetilde{XC}_{e,j }\)) are considered uncertain due to traffic risks. Moreover, \(\eta_{n,h}^{n} , \eta_{n,o}^{ 'n} , \eta_{o,i}^{o} , \eta_{i,e}^{i} , \eta_{e,j}^{e}\) reflect described variables corresponding to the constraints of the robust-box linear optimization. The investigated deterministic model is extended to the robust optimization model as follows:
Subject to:
and constraints (3)–(11), (13)–(26), (28)–(31).
The objective function (1) is converted to (33), and constraints (34)–(46) are added based on the Box-uncertainty approach.
2.3 Reliable model
Subject to:
Rights and permissions
About this article
Cite this article
Hosseini-Motlagh, SM., Gilani Larimi, N. & Oveysi Nejad, M. A qualitative, patient-centered perspective toward plasma products supply chain network design with risk controlling. Oper Res Int J 22, 779–824 (2022). https://doi.org/10.1007/s12351-020-00568-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12351-020-00568-4