Abstract
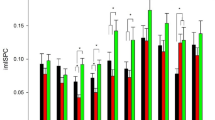
The theoretical foundation of brain-computer interface partly lies in the neural mechanism of motor function plasticity. There has been extensive research on the functional neuroplasticity induced by motor skill training in the human cerebral cortex; however, less is known about the specifics within the cerebellum. The present study employed resting-state functional magnetic resonance imaging (fMRI) data from athletes and matched non-athlete controls to investigate the adaptation of cerebellar functional segregation and integration in athletes who require rapid visual-motor coordination. First, this study utilized a data-driven blind-deconvolution hemodynamic response functions (HRF) retrieval technique to estimate voxel-wise HRF that represent local functional segregation. Second, the study quantified effective connectivity using conditional Granger causality (CGC) analysis as a means of characterizing directed functional integration. Lastly, the logistic regression classification model was applied to evaluating the importance of those significant features in two groups’ comparison. The athletes exhibited greater HRF response heights in the visual-spatial cognitive regions, but lower excitatory/inhibitory effects between these regions and the motor execution areas in the cerebellum when compared to the control group. These findings suggested that there was improved local functional segregation within the visual-spatial cognitive regions, as well as reduced functional integration between these regions and the motor execution areas in the cerebellum among athletes. Our results suggested the antagonistic alterations of cerebellar functional segregation and integration induced by motor skill training, and consequently to accelerate the reaction, movement planning, and execution in athletes who required fast demands of visual-motor coordination. Our findings shed new light on how motor skill training drove neuroplasticity within the cerebellum and offered a deeper understanding of the complementary hypotheses of neural efficiency and neural proficiency that underlay optimal athletic performance.





Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Gomez-Pilar J, Corralejo R, Nicolas-Alonso LF, Álvarez D, Hornero R. Neurofeedback training with a motor imagery-based BCI: neurocognitive improvements and EEG changes in the elderly. Med Biol Eng Compu. 2016;54(11):1655–66.
Gomez-Pilar J, Corralejo R, Nicolas-Alonso LF, Álvarez D, Hornero R. Assessment of neurofeedback training by means of motor imagery based-BCI for cognitive rehabilitation. Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual International Conference. 2014;2014:3630–3.
Abiri R, Borhani S, Sellers EW, Jiang Y, Zhao X. A comprehensive review of EEG-based brain-computer interface paradigms. J Neural Eng. 2019;16(1): 011001.
Mridha MF, Das SC, Kabir MM, Lima AA, Islam MR, Watanobe Y. Brain-computer interface: advancement and challenges. Sensors (Basel, Switzerland). 2021;21(17).
Gao Q, Yu Y, Su X, Tao Z, Zhang M, Wang Y, et al. Adaptation of brain functional stream architecture in athletes with fast demands of sensorimotor integration. Hum Brain Mapp. 2019;40(2):420–31.
Tao Z, Gao Q, Yu Y, Chen H. A study of brain plasticity of small-ball players based on ReHo method. Journal of Chengdu Sport University. 2017;43(06):98–102.
Shao M, Lin H, Yin D, Li Y, Wang Y, Ma J, et al. Learning to play badminton altered resting-state activity and functional connectivity of the cerebellar sub-regions in adults. PLoS ONE. 2019;14(10): e0223234.
Pezoulas VC, Michalopoulos K, Klados MA, Micheloyannis S, Bourbakis NG, Zervakis M. Functional connectivity analysis of cerebellum using spatially constrained spectral clustering. IEEE J Biomed Health Inform. 2019;23(4):1710–9.
Sereno MI, Diedrichsen J, Tachrount M, Testa-Silva G, d’Arceuil H, De Zeeuw C. The human cerebellum has almost 80% of the surface area of the neocortex. Proc Natl Acad Sci USA. 2020;117(32):19538–43.
Balsters JH, Cussans E, Diedrichsen J, Phillips KA, Preuss TM, Rilling JK, et al. Evolution of the cerebellar cortex: the selective expansion of prefrontal-projecting cerebellar lobules. Neuroimage. 2010;49(3):2045–52.
Barton RA, Venditti C. Rapid evolution of the cerebellum in humans and other great apes. Curr Biol. 2014;24(20):2440–4.
Kochiyama T, Ogihara N, Tanabe HC, Kondo O, Amano H, Hasegawa K, et al. Reconstructing the Neanderthal brain using computational anatomy. Sci Rep. 2018;8(1):6296.
Di X, Zhu S, Jin H, Wang P, Ye Z, Zhou K, et al. Altered resting brain function and structure in professional badminton players. Brain connectivity. 2012;2(4):225–33.
Li W, Kong X, Ma J. Effects of combat sports on cerebellar function in adolescents: a resting-state fMRI study. The British journal of radiology. 2021:20210826.
Friston KJ, editor Introduction experimental design and statistical parametric mapping 2003.
Friston KJ, Frith CD, Frackowiak RSJ. Time-dependent changes in effective connectivity measured with PET. 1993;1(1):69–79.
Geweke JF. Measures of conditional linear dependence and feedback between time series. J Am Stat Assoc. 1984;79(388):907–15.
Ding M, Chen Y, Bressler SL. Granger causality: basic theory and application to neuroscience. Handbook of Time Series Analysis. 2006. p. 437–60.
Deshpande G, LaConte S, James GA, Peltier S, Hu X. Multivariate Granger causality analysis of fMRI data. Hum Brain Mapp. 2009;30(4):1361–73.
Jiao Q, Lu G, Zhang Z, Zhong Y, Wang Z, Guo Y, et al. Granger causal influence predicts BOLD activity levels in the default mode network. 2011;32(1):154–61.
Gao Q, Duan X, Chen H. Evaluation of effective connectivity of motor areas during motor imagery and execution using conditional Granger causality. Neuroimage. 2011;54(2):1280–8.
Gao Q, Zou K, He Z, Sun X, Chen H. Causal connectivity alterations of cortical-subcortical circuit anchored on reduced hemodynamic response brain regions in first-episode drug-naïve major depressive disorder. Sci Rep. 2016;6:21861.
Wu GR, Liao W, Stramaglia S, Ding JR, Chen HF, Marinazzo D. A blind deconvolution approach to recover effective connectivity brain networks from resting state fMRI data. Med Image Anal. 2013;17(3):365–74.
Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–7.
Lindquist MA, Wager TD. Validity and power in hemodynamic response modeling: a comparison study and a new approach. Hum Brain Mapp. 2007;28(8):764–84.
Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113.
Malekpour S, Sethares WA. Conditional Granger causality and partitioned Granger causality: differences and similarities. Biol Cybern. 2015;109(6):627–37.
Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19(4):1273–302.
Ji J, Liu J, Liang P, Zhang A. Learning effective connectivity network structure from fMRI data based on artificial immune algorithm. PLoS ONE. 2016;11(4): e0152600.
Handwerker DA, Ollinger JM, D’Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage. 2004;21(4):1639–51.
Wu GR, Marinazzo D. Retrieving the hemodynamic response function in resting state fMRI: methodology and applications. PeerJ PrePrints. 2015;3:6050–3.
Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–45.
Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101(9):3316–21.
Talukdar T, Nikolaidis A, Zwilling CE, Paul EJ, Hillman CH, Cohen NJ, et al. Aerobic fitness explains individual differences in the functional brain connectome of healthy young adults. Cerebral cortex (New York, NY : 1991). 2018;28(10):3600–9.
King M, Hernandez-Castillo CR, Poldrack RA, Ivry RB, Diedrichsen J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat Neurosci. 2019;22(8):1371–8.
Schmahmann JD. The cerebellum and cognition. Neurosci Lett. 2019;688:62–75.
Stoodley CJ, Valera EM, Schmahmann JD. An fMRI study of intra-individual functional topography in the human cerebellum. Behav Neurol. 2010;23(1–2):65–79.
Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59(2):1560–70.
Schmahmann JD, Guell X, Stoodley CJ, Halko MA. The theory and neuroscience of cerebellar cognition. Annu Rev Neurosci. 2019;42:337–64.
Zang Z, Yan C, Dong Z, Huang J, Zang Y. Granger causality analysis implementation on MATLAB: a graphic user interface toolkit for fMRI data processing. J Neurosci Methods. 2012;203(2):418–26.
Guo Z, Li A, Yu L. “Neural efficiency” of athletes’ brain during visuo-spatial task: an fMRI study on table tennis players. Front Behav Neurosci. 2017;11:72.
Wolf S, Brölz E, Scholz D, Ramos-Murguialday A, Keune PM, Hautzinger M, et al. Winning the game: brain processes in expert, young elite and amateur table tennis players. Front Behav Neurosci. 2014;8:370.
Li L, Smith DM. Neural efficiency in athletes: a systematic review. Front Behav Neurosci. 2021;15: 698555.
Dietrich A. Transient hypofrontality as a mechanism for the psychological effects of exercise. Psychiatry Res. 2006;145(1):79–83.
Filho E, Dobersek U, Husselman TA. The role of neural efficiency, transient hypofrontality and neural proficiency in optimal performance in self-paced sports: a meta-analytic review. Exp Brain Res. 2021;239(5):1381–93.
Holmes PS, Wright DJ. Motor cognition and neuroscience in sport psychology. Curr Opin Psychol. 2017;16:43–7.
Bertollo M, Doppelmayr M, Robazza C. Using brain technologies in practice. Handbook Sport Psychol. 2020;666–93.
Funding
This study was funded by the Ministry of Science and Technology of China (2021ZD0201701 and 2018AAA0100705), the National Natural Science Foundation of China (62173070, 82121003, 62036003, U1808204, 82072006, and 61906034), and the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-D-202003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the research ethical committee of School of Life Science and Technology, University of Electronic Science and Technology of China and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, W., Wu, J., Li, Y. et al. The Antagonistic Alterations of Cerebellar Functional Segregation and Integration in Athletes with Fast Demands of Visual-Motor Coordination. Cogn Comput 15, 1813–1824 (2023). https://doi.org/10.1007/s12559-023-10150-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12559-023-10150-7