Abstract
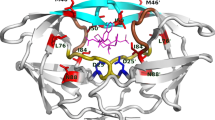
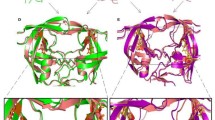
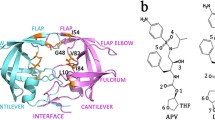
Amprenavir is an HIV-1 protease inhibitor (PI) that has recently been approved for the treatment HIV/AIDS. Despite its outstanding safety and efficacy, site-specific mutations occurring at one or more residues in HIV-1 protease have caused the development of resistance to PI. Unfortunately, a comprehensive understanding of the resistance mechanisms is still lacking. Therefore, the present investigation aims to uncover the mechanism behind the resistance for amprenavir to HIV-1 protease triple mutant (V32I, I47V and V82I) by computational techniques. We have also highlighted the effect of mutations on the binding site residues and flap comprising residues in the HIV-1 protease by means of flexibility analysis. Molecular dockings were performed to gain insights into the binding mode of the amprenavir with HIV-1 protease structure. Subsequently, the docking results were also validated by means of PEARLS program. The obtained results provide a detailed explanation of the resistance caused by triple mutant (V32I, I47V and V82I) and may give imperative clue for the design of drugs to combat amprenavir resistance.




Similar content being viewed by others
References
Baldwin ET, Bhat TN, Liu B, Pattabiraman N, Erickson JM (1995) Emergence of protease inhibitor resistance mutations in human immunodeficiency virus type 1 isolates from patients and rapid screening procedure for their detection. Nat Struct Biol 2:244–249
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne E (2000) The protein data bank. Nucleic Acids Res 28:235–242
Brunton LL, Lazo JS, Parker KL (2006) Goodman and Gilmans’s the pharmacological basis of therapeutic, 11th edn. McGraw Hill, USA
Cavasotto CN, Kovacs JA, Abagyan RA (2005) Representing receptor flexibility in ligand docking through relevant normal modes. J Am Chem Soc 127(26):9632–9640
Connolly ML (1983) Analytical molecular surface calculation. J Appl Crystallogr 16:548–558
Csaba M, Gromiha MM, Gerard P, Gábor ET, István S (2005) SRide: a server for identifying stabilizing residues in proteins. Nucleic Acids Res 33:W303–W305
Debouck C, Gorniak JG, Strickler JE, Meek TD, Metcalf BW, Rosenberg M (2006) Human immunodeficiency virus protease expressed in Escherichia coli exhibits autoprocessing and specific maturation of the gag precursor. Proc Natl Acad Sci USA 84(24):8903–8906
Duhovny D, Nussinov R, Wolfson HJ (2002) Lecture notes in computer science, vol 2452. Springer, New York, pp 185–200
Feldman J, Snyder KA, Ticoll A, Pintilie G, Hogue CW (2006) A complete small molecule dataset from the protein data bank. FEBS Lett 580:1649–1653
Freeman S, Herron JC (2007) Evolutionary analysis. A case for evolutionary thinking: understanding HIV, 4th edn. Pearson Benjamin Cummings, San Francisco
Gasteiger J, Rudolph C, Sadowski J (1990) Automatic generation of 3D-atomic coordinates for organic molecules. Tetrahedron Comput Meth 3:537–547
Han LY, Lin HH, Li ZR, Zhena CJ, Cao ZW, Xie B, Chen YZ (2006) PEARLS: program for energetic analysis of receptor–ligand system. J Chem Inf Model 16:445–450
Hess B, Kutzner C, Spoel D, Lindahl E (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4(3):435–447
Hinkle A, Tobacman LS (2003) Folding and function of the troponin tail domain Effects of cardiomyopathic troponin T mutations. J Biol Chem 278:506–513
Kim EE, Baker CT, Dwyer MD, Murcko MA, Rao BG, Tung RD, Navia MA (1995) Crystal structure of HIV-1 protease in complex with VX-478, a potent and orally bioavailable inhibitor of the enzyme. J Am Chem Soc 117:1181–1182
Louis JM, Weber IT, Tozser J, Clore GM, Gronenborn AM (2000) HIV-1 protease: maturation, enzyme specificity, and drug resistance. Adv Pharmacol 49:111–146
Martinez-Cajas JL, Wainberg MA (2007) Protease inhibitor resistance in HIV-infected patients: molecular and clinical perspectives. Antiviral Res 76(3):203–221
Nancy EK, Emilio AE, William AS, Lenora JD, Jill CH (1988) Active human immunodeficiency virus protease is required for viral infectivity. Proc Nati Acad Sci USA 85:4686–4690
Parkin NT, Chappey C, Petropoulos CJ (2003) Improving lopinavir genotype algorithm through phenotype correlations: novel mutation patterns and amprenavir cross-resistance. AIDS 17:955–961
Parthasarathy S, Murthy MR (2000) Protein thermal stability: insights. Protein Eng 13:9–13
Rajasekaran R, George Priya Doss C, Sudandiradoss C, Ramanathan K (2008) Effect of deleterious nsSNP on the HER2 receptor based on stability and binding affinity with herceptin: a computational approach. C R Biol 331:409–417
Randolph JT, DeGoey DA (2004) Peptidomimetic inhibitors of HIV protease. Curr Top Med Chem 4(10):1079–1095
Ringe D, Petsko GA (1986) Study of protein dynamics by X-ray diffraction. Methods Enzymol 131:389–433
Rose RE, Gong YF, Greytok JA, Bechtold CM, Terry BJ, Robinson BS, Alam M, Colonno RJ, Lin PF (1996) Human immunodeficiency virus type 1 viral background plays a major role in development of resistance to protease inhibitors. Proc Natl Acad Sci USA 93(4):1648–1653
Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ (2005) PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res 33:363–367
Shen ChenHsiang, Wang YuanFang, Andrey YK, Robert WH (2010a) Amprenavir complexes with HIV-1 protease and its drug. FEBS J 277(18):3699–3714
Shen CH, Wang YF, Kovalevsky AY, Harrison RW, Weber IT (2010b) Amprenavir complexes with HIV-1 protease and its drug-resistant mutants altering hydrophobic clusters. FEBS J 277:3699–3714
Spinelli S, Liu QZ, Alzari PM, Hirel PH, Poljak RJ (1991) The three-dimensional structure of the aspartyl protease from the HIV-1 isolate BRU. Biochimie 73:1391–1396
Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJ (2005) GROMACS: fast, flexible, and free. Comput Chem 26(16):1701–1718
Suhre K, Sanejouand YH (2004) ElNemo: a normal mode web server for protein movement analysis and the generation of templates for molecular replacement. Nucleic Acids 32:610–614
Tie Y, Wang YF, Boross PI, Chiu TY, Ghosh AK, Tozser J, Louis JM, Harrison RW, Weber IT (2012) Critical differences in HIV-1 and HIV-2 protease specificity for clinical inhibitors. Protein Sci 21:339–350
Wallace AC, Laskowski RA, Thornton JM (1995) LIGPLOT: a program to generate schematic diagrams of protein–ligand interactions. Protein Eng 8(2):127–134
Weber IT, Kovalevsky AY, Harrison RW (2007) Frontiers in drug design & discovery. Bentham Science Publishers, IL, p 3
Yuan Z, Bailey TL, Teasdale RD (2005) Prediction of protein. Proteins 58:905–912
Yunfeng T, Yuan-Fang W, Peter IB, Ting-Yi C, Arun KG, Jozsef T, John M, Robert WH, Irene TW (2012) Critical differences in HIV-1 and HIV-2 protease specificity for clinical inhibitors. Protein Sci 21(3):339–350
Zhang C, Vasmatzis G, Cornette JL, DeLisi C (1997) Determination of atomic desolvation energies from the structures of crystallized proteins. J Mol Biol 267:707–726
Acknowledgments
The authors express deep sense of gratitude to the management of Vellore Institute of Technology for all the support, assistance and constant encouragements to carry out this work. The authors also thank reviewers for their valuable comments and suggestion in the improvement of our manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramanathan, K., Shanthi, V., Pratik, U. et al. Computational analysis of amprenavir resistance triple mutant (V32I, I47V and V82I) in HIV-1 protease. Netw Model Anal Health Inform Bioinforma 3, 48 (2014). https://doi.org/10.1007/s13721-014-0048-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13721-014-0048-z