Abstract
Purpose
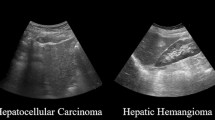
Ultrasound image acquisition has the advantages of being low cost, rapid, and non-invasive, and it does not produce radiation. Currently, ultrasound is widely used in the diagnosis of liver tumors. However, owing to the complex presentation and diverse features of benign and malignant liver tumors, accurate diagnosis of liver tumors using ultrasound is difficult even for experienced radiologists. In recent years, artificial intelligence-assisted diagnosis has proven to provide effective support to radiologists. However, there is room for further improvement in the existing ultrasound artificial intelligence diagnostic model of liver tumor. First, the image diagnostic model may not fully consider relevant clinical data in the decision-making process. Second, owing to the difficulty in collecting biopsy pathology and physician-labeled ultrasound data of liver tumors, training datasets are usually small, and commonly used large neural networks tend to overfit on small datasets, which seriously affects the generalization of the model.
Methods
In this study, we propose a deep learning–assisted diagnosis model called USC-ENet, which integrates B-mode ultrasound features of liver tumors and clinical data of patients, and we design a small neural network specifically for small-scale medical images combined with an attention mechanism.
Results and conclusion
Real data from 542 patients with liver tumors (N = 2168 images) are used during model training and validation. Experiments show that USC-ENet can achieve a good classification effect (area under the curve = 0.956, sensitivity = 0.915, and specificity = 0.880) after small-scale data training, and it has certain interpretability, showing good potential for clinical adoption. In conclusion, our model provides not only a reliable second opinion for radiologists but also a reference for junior radiologists who lack clinical experience.








Similar content being viewed by others
Data & Code availability
The datasets and code generated during the current study are available from the corresponding author on reasonable request
Notes
The datasets and code generated during the current study are available from the corresponding author on reasonable request.
References
Li G, Le Y, Peng Yu, et al. Research progress on sensitivity related factors of sorafenib in the treatment of liver cancer. J Hepatobiliary Surg. 2018;26(1):74–7.
Zhang D, Jiang F, Zhifang H, et al. Prevention of primary liver cancer. Chin J Gerontol. 2018;38(17):4317–9.
Cai L. Application of contrast-enhanced ultrasound in early diagnosis of cirrhosis complicated with small hepatocellular carcinoma. Imag Res Med Appl. 2018;2(5):91–3.
Liu S, Wang Y, Yang X, Lei B, Liu L, Li S Xiang, Ni D, Wang T. Deep learning in medical ultrasound analysis: a review. Engineering. (2018). https://doi.org/10.1016/j.eng.2018.11.020.
Buda M, Wildman-Tobriner B, Hoang JK, et al. Management of thyroid nodules seen on US images: deep learning may match performance of radiologists. Radiology. 2019;292(3):695–701.
Cao Z, Duan L, Yang G, et al. Breast tumor detection in ultrasound images using deep learning. In: Proceedings of international workshop on patch-based techniques in medical imaging. 2017. pp. 121–128.
Wang K, Lu X, Zhou H, et al. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut. 2019;68(4):729–41.
Xi IL, Wu J, Guan J, et al. Deep learning for differentiation of benign and malignant solid liver lesions on ultrasonography. Abdom. Radiol. 2020. https://doi.org/10.1007/s00261-020-02564-w.
Hu HT, Wang W, et al. Artificial intelligence assists identifying malignant versus benign liver lesions using contrast-enhanced ultrasound. Gastroenterol Hepatol. 2021. https://doi.org/10.1111/jgh.15522.
Zhen S, Cheng M, Tao Y, et al. Deep learning for accurate diagnosis of liver tumor based on magnetic resonance imaging and clinical data. Front Oncol. 2020. https://doi.org/10.3389/fonc.2020.00680
Yang Q, Wei J, Hao X, et al. Improving B-mode ultrasound diagnostic performance for focal liver lesions using deep learning: a multicentre study. EBioMedicine. 2020;56:102777.
Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175(2):313–26.
Tzutalin DL. GitHub repository. https://github.com/tzutalin/labelImg Accessed Dec 2017.
Jia D, Wei D, Socher R, et al. ImageNet: a large-scale hierarchical image database. In: Proceedings of IEEE computer vision & pattern recognition (CVPR). 2009. pp. 248–255.
bahdanau D, Cho K, Bengio Y. Neural machine translation by jointly learning to align and translate. In: Proceedings of international conference on learning representations (ICLR). 2014. arXiv:1409.0473
Yang J, Zhang D, Frangi AF, et al. Two-dimensional PCA: a new approach to appearance-based face representation and recognition. IEEE Trans Pattern Anal Mach Intell. 2004;26(1):131–7.
Hu J, Shen L, Sun G. Squeeze-and-excitation networks. In: Proceedings of IEEE computer vision & pattern recognition (CVPR). 2018. pp. 7132–7141.
LaValley MP. Logistic regression. Circulation. 2008;117(18):2395–9.
Myung IJ. Tutorial on maximum likelihood estimation. J Math Psychol. 2003;47(1):90–100.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics, to be published. https://doi.org/10.2307/2531595
Paszke A, et al. Pytorch: an imperative style, high-performance deep learning library. Adv Neural Inform Process Syst. 2019. arXiv:1912.01703
Sanders J, Kandrot E. CUDA by example: an introduction to general-purpose GPU programming. In: Addison-Wesley Professional. 2010.
He K, Zhang X, Ren S, et al. Deep residual learning for image recognition. In: Proceedings of IEEE computer vision & pattern recognition (CVPR). 2016. pp. 770–778.
Simonyan K, Zisserman A. Very deep convolutional networks for large-scale image recognition. 2014. arXiv:1409.1556
Howard AG, et al. Mobilenets: efficient convolutional neural networks for mobile vision applications. 2017. arXiv:1704.04861
Ke A, Ellsworth W, Banerjee O, et al. CheXtransfer: performance and parameter efficiency of ImageNet models for chest X-Ray interpretation. In: Proceedings of the conference on health, inference, and learning (CHIL). 2021. pp. 116–124.
Rigatti SJ. Random forest. J Insur Med. 2017;47(1):31–9.
Frerichs FT. A clinical treatise on diseases of the liver. In: New Sydenham Society; 1861.
Wang H, Wang Z, Du M, et al. Score-CAM: score-weighted visual explanations for convolutional neural networks. In: Proceedings of the IEEE/CVF conference on computer vision and pattern recognition workshops; 2020. pp. 24–25
Yang J. Progress in diagnosis and treatment of liver abscess. Chin J Pract Surg. 2003;23(011):693–4.
Li S, Wang J, Yan Z, et al. Interventional therapy of metastatic liver cancer and its influencing factors. J Med Imag. 2001. https://doi.org/10.1155/2021/3392433
Marchuk DA. Pathogenesis of hemangioma. J Clin Investig. 2001;107(6):665–6.
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. The Lancet. https://doi.org/10.1016/S0140-6736(11)61347-0
Mazurowski MA, Habas PA, Zurada JM, et al. Training neural network classifiers for medical decision making: The effects of imbalanced datasets on classification performance. Neural Netw. 2008;21(2):427–36.
Tan, Mingxing, and Quoc Le. "Efficientnet: Rethinking model scaling for convolutional neural networks." International conference on machine learning. PMLR, 2019.
Bryman, Alan, and Duncan Cramer. Quantitative data analysis with minitab: A guide for social scientists. Routledge, 2003.
Funding
This study was funded by Yunnan Provincial Science and Technology Department(202201AY070001)
Author information
Authors and Affiliations
Contributions
Tingting Zhao developed the methods and performed the statistical analysis; Tao Feng and Rui Bu designed and supervised the study, Zhiyong Zeng revised the manuscript, and Wenjing Tao, Tong Li, and Xing Yu collected the datasets. All authors contributed to the writing and the interpretation of the results
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Ethical approval
This research was approved by the authors’ Institutional Review Board (Medical Ethics Committee of the Second Affiliated Hospital of Kunming Medical University)
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, T., Zeng, Z., Li, T. et al. USC-ENet: a high-efficiency model for the diagnosis of liver tumors combining B-mode ultrasound and clinical data. Health Inf Sci Syst 11, 15 (2023). https://doi.org/10.1007/s13755-023-00217-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13755-023-00217-y