Abstract
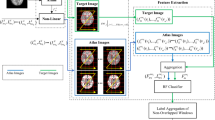
The brain image segmentation in magnetic resonance imaging (MRI) is essential to smooth the pathway to the data of outcome measures clinically. This paper proposes a new framework built on graph structure modeling using Brain-delineation Registration through Configuration of Inner Shape for Segmentation (BR-CISS) qualitatively in color channels of Pink, Blue, Green, and Red space (PBGRs) to track the development of baby brain tissue quantitatively in MRI. The BR-CISS starts with the linear intensity normalization for contract augmentation, followed by brain-delineation registration via the inner shape configuration. The next graph structure modeling is to convert registered images into the PBGRs model. In the segmentation process, the minimal mean squared error are used as the color matching criterion to segment white matter (WM) in P channel, gray matter (GM) in B channel, cerebrospinal fluid (CSF) in G channel, and the background in R channel. In addition, the calculation of actual numbers of voxels quantitatively is accomplished by binary image transformation along with pattern sketch map. The BR-CISS is implemented in the dataset of baby brain MR images during the first 5 years of life. Results show the capability of the proposed framework for both qualitative segmentation and quantitative measures of baby MR brain tissue with graph-based vision in PBGRs. This study found that (a) the sum of WM and GM peaked around about the age of 4; (b) GM peaked around the age of 3; (c) WM kept a steady growth from around the age of 1 thereafter. The proposed BR-CISS is the first study to track the development of baby brain tissue quantitatively jointly with the segmentation of the brain MR images in the PBGRs qualitatively during the first 5 years of life. Taking the advantage of the graph structure modeling with color information in the PBGRs, this study has made the effort in the direction for providing some new insight into the maturation of the WM, the GM, and the CSF patterns graphically in the early life in the brain MR images.








Similar content being viewed by others
References
Brain Development Cooperative Group, Evans A. C. The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202.
Bishop CM. Pattern recognition and machine learning. NY: Springer; 2006.
Chen Y-J, Liu C-M, Hsu Y-C, Lo Y-C, Hwang T-J, Hwu H-G, Lin Y-T, Tseng W-YI. Individualized prediction of schizophrenia based on the whole-brain pattern of altered white matter tract integrity. Hum Brain Mapp. 2018;39:575–87.
Descoteaux M, Collins DL, Siddiqi K. A geometric flow for segmenting vasculature in proton-density weighted MRI. Med Image Anal. 2008;12:497–513.
Duchesne S, Caroli A, Geroldi C, Collins DL, Frisoni GB. Relating one-year cognitive change in mild cognitive impairment to baseline MRI features. Neuroimage. 2009;47:1363–70.
Devi CN, Chandrasekharan A, Sundararaman VK, Alex ZC. Neonatal brain MRI segmentation: a review. Comput Biol Med. 2015;64:163–78.
Demsar J. Statistical comparisons of classifiers over multiple data sets. J Mach Learn Res. 2006;7:1–30.
Drozdzal M, Vorontsov E, Chartrand G, Kadoury S, Pal C, et al. The importance of skip connections in biomedical image segmentation. In: Carneiro G, et al., editors. Deep learning and data labeling for medical applications. Springer; 2016. p. 179–87.
Duyn JH. The future of ultra-high field MRI and fMRI for study of the human brain. Neuroimage. 2012;62:1241–8.
Egger J, Kapur T, Fedorov A, Pieper S, Miller JV, Veeraraghavan H, Freisleben B, Golby AJ, Nimsky C, Kikinis R. GBM volumetry using the 3D slicer medical image computing platform. Sci Rep. 2013;1364:1–7.
Emerson, RW, Adams C, Nishino T, Hazlett HC, and et al. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci Transl Med. 2017;9:1–8.
Fonov V, Evans A, Botteron K, Almli CR, McKinstry RC, Collins DL, et al. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54:313–27.
Frydrychowicz A, Lubner MG, Brown JJ, Merkle EM, Nagle SK, Rofsky NM, Reeder SB. Hepatobiliary MR imaging with gadolinium-based contrast agents. J Magn Reson Imaging. 2012;35:492–511.
Fukunaga K. Introduction to statistical pattern recognition. Boston: Academic Press; 1990.
Guo P, Evans A, Bhattacharya P. Nuclei segmentation for quantification of brain tumors in digital pathology images”. J Software Sci Comput Intell. 2018;10:36–49.
Guo P. A tissue-based biomarker model for predicting disease patterns. J Knowl Based Sys. 2017;276:160–9.
Guo, P. A clinical measuring platform for building the bridge across the quantification of pathological N-cells in medical imaging for studies of disease. MICCAI 2019 on Clinical Image-based Procedures, Shenzhen, China; the Proceedings appeared in the series Lecture Notes in Comput. Sci., LNCS 11840, Springer, pp. 85–93, 2019.
Gui L, Lisowski R, Faundez T, Huppi PS, Lazeyras F, Kocher M. Morphology-driven automatic segmentation of MR images of the neonatal brain. Med Image Anal. 2012;16:1565–79.
Goshtasby AA. Image registration: principles, tools and methods. Springer; 2012.
Hazlett HC, Gu H, McKinstry RC, Shaw DWW, and for the IBIS Network, et al. Brain volume findings in 6-month-old infants at high familial risk for autism. Am J Psychiatry. 2012;169:601–8.
Hyde KL, Samson F, Evans AC, Mottron L. Neuroanatomical differences in brain areas implicated in perceptual and other core features of autism revealed by cortical thickness analysis and voxel-based morphometry. Hum Brain Mapp. 2010;31:556–66.
Haykin SO. Adaptive filter theory. 5th ed. Prentice Hall; 2013.
Isensee F, Jaeger PF, Kohl SAA, Petersen J, Maier-Hein KH. nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation. Nat Methods. 2021;18:203–11.
Lewis JD, Evans A, Pruett J, Botteron K, Zwaigenbaum L, et al. Network inefficiencies in autism spectrum disorder at 24 months. Transl Psychiatry. 2014;4:1–11.
Lebel C, Deoni S. The development of brain white matter microstructure. Neuroimage. 2018;182:207–18.
Martínez-Murcia FJ, Górriz JM, Ramírez J, Ortiz A. A structural parametrization of the brain using hidden Markov models based paths in Alzheimer’s disease. J Neural Syst. 2016;26:1–18.
Kruper J, Yeatman JD, Richie-Halford A, Bloom D, Grotheer M, Caffarra S, Kiar G, Karipidis II, Roy E, Chandio BQ, Garyfallidis E, Rokem A. Evaluating the reliability of human brain white matter tractometry. Aperture Neuro. 2021;1:1–25.
Khundrakpam B, Vainik U, Gong J, Al-Sharif N, Bhutani N, Kiar G, Zeighami Y, Kirschner M, Luo C, Dagher A, Evans A. Neural correlates of polygenic risk score for autism spectrum disorders in general population. Brain Commun. 2020;2:1–13.
Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. NeuroReport. 2007;18:23–7.
Moon TK, Stirling WC. Mathematical methods and algorithms for signal processing. Prentice Hall; 2000.
Petrou M, Bosdogianni P. Image processing the fundamentals. UK: Wiley; 2004.
Rafael RC, Wood RE. Digital image processing. 3rd ed. Upper Saddle River, NJ: Prentice Hall; 2008.
Rane P, Cochran D, Hodge SM, Haselgrove C, Kennedy DN, Frazier JA. Connectivity in autism: a review of MRI connectivity studies. Harv Rev Psychiatry. 2015;23:223–44.
Shen MD, Swanson MR, Wolff JJ, Elison JT, and for the IBIS Network, et al. Subcortical brain development in autism and fragile X syndrome: evidence for dynamic, age- and disorder-specific trajectories in infancy. Am J Psychiatry. 2022. https://doi.org/10.1176/appi.ajp.21090896).
Shen MD, Kim SH, McKinstry RC, Gu H, and for the IBIS Network, et al. Increased extra-axial cerebrospinal fluid in high-risk infants who later develop autism. Biol Psychiatry. 2017;82:186–93.
Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–9.
Shi F, Fan Y, Tang S, Gilmore JH, Lin W, Shen D. Neonatal brain image segmentation in longitudinal MRI studies. Neuroimage. 2010;49:391–400.
So RWK, Tang TWH, Chung ACS. Non-rigid image registration of brain magnetic resonance images using graph-cuts. Pattern Recogn. 2011;44:2450–67.
Tohka J. Partial volume effect modeling for segmentation and tissue classification of brain magnetic resonance images: a review. World J Radiol. 2014;11:855–64.
Vasung L, Turk EA, Ferradal SL, Sutin J, Stout JN, Ahtam B, Lin P-Y, Grant PE. Exploring early human brain development with structural and physiological neuroimaging. Neuroimage. 2019;187:226–54.
Wang L, Shi F, Lin W, Gilmore JH, Shen D. Automatic segmentation of neonatal images using convex optimization and coupled level sets. Neuroimage. 2011;108:805–17.
Wang Y. The cognitive process and formal models of human attentions. Int J Softw Sci Comput Intell. 2013;7:32–50.
Willcocks CG, Jackson PTG, Nelson CJ, Nasrulloh AV, Obara B. Interactive GPU active contours for segmenting inhomogeneous objects. J Real Time Image Process. 2019;7:2305–18.
Wang J, Vachet C, Rumple A, Gouttard S, Ouziel C, Perrot E, Du G, Huang X, Gerig G, Styner M. Multi-atlas segmentation of subcortical brain structures via the AutoSeg software pipeline. Front Neuroinform. 2014. https://doi.org/10.3389/fninf.2014.00007.
West J, Warntjes JB, Lundberg P. Novel whole brain segmentation and volume estimation using quantitative MRI. Eur Radiol. 2012;22:998–1007.
Zitová B, Flusser J. Image registration methods: a survey. Image Vision Comput. 2003;21:977–1000.
Acknowledgements
The author would like to express thanks to the anonymous reviewers for their time, valuable comments, and useful suggestions for the improvement of this manuscript.
Funding
No funding was received for this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that she has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, P. Tracking the Development of Baby Brain Tissue with Color Vision in Magnetic Resonance Imaging. SN COMPUT. SCI. 3, 266 (2022). https://doi.org/10.1007/s42979-022-01151-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42979-022-01151-8