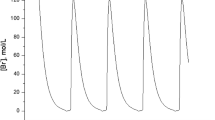
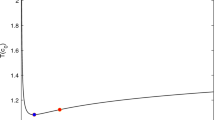
Abstract
The paper proposes a systematic method for fixed-point bifurcation analysis in circadian cells and similar biological models using interval polynomials theory. The stages for performing fixed-point bifurcation analysis in such biological systems comprise (i) the computation of fixed points as functions of the bifurcation parameter and (ii) the evaluation of the type of stability for each fixed point through the computation of the eigenvalues of the Jacobian matrix that is associated with the system’s nonlinear dynamics model. Stage (ii) requires the computation of the roots of the characteristic polynomial of the Jacobian matrix. This problem is nontrivial since the coefficients of the characteristic polynomial are functions of the bifurcation parameter and the latter varies within intervals. To obtain a clear view about the values of the roots of the characteristic polynomial and about the stability features they provide to the system, the use of interval polynomials theory and particularly of Kharitonov’s stability theorem is proposed. In this approach, the study of the stability of a characteristic polynomial with coefficients that vary in intervals is equivalent to the study of the stability of four polynomials with crisp coefficients computed from the boundaries of the aforementioned intervals. The efficiency of the proposed approach for the analysis of fixed-point bifurcations in nonlinear models of biological neurons is tested through numerical and simulation experiments.





Similar content being viewed by others
References
Aqil M, Hong KS, Jeong MY (2012) Synchronization of coupled chaotic FitzHugh–Nagumo systems. Commun Nonlinear Sci Numer Simul 17:1615–1627
Baird Emertrout G, Terman DH (2009) Series in interdisciplinary applied Mathematics. Mathematical foundations of neuroscience. Springer, Berlin
Chen G, Moiola JL, Wang HO (2000) Bifurcation control: theories methods and applications. Int J Bifurc Chaos 10(3):511–548
Clairambault J (2006) Physiologically based modelling of circadian control on cell proliferation. In: 28th IEEE EMBS annual international conference, New York, USA, Sep 2006
Clairambault J (2008) A step toward optimization of cancer therapeutics: physiologically based modeling of circadian control on cell proliferation. IEEE Eng Med Biol Mag 27(1):20–24
Dorak RO (2013) Control of repetitive firing in Hodgkin–Huxley nerve fibers using electric fields. Chaos, Solitons and Fractals 52:66–72
Duan H, Cai C, Han C, Che Y (2012) Bifurcation control in Morris–Lecar neuron model via washout filter with a linear term based on filter-aided dynamic feedback. Adv Mater Res 485:600–603
Efimov DV, Fradkov AL (2006) Adaptive tuning to bifurcation for time-varying nonlinear systems. Automatica 42:417–425
Francois P (2005) A model for the Neurospora circadian clock. Biophys J 88:2369–2383
Gerstner W, Kistler W (2002) Spiking neuron models: single neurons, populations, plasticity. Cambridge University Press, Cambridge
Gonze D, Leloup JC, Goldbeter A (2000) Theoretical models for circadian rhythms in Neurospora and Drosophila. C R Acad Sci Paris, Sciences de la Vie / Life Sciences 323:57–67
Gonze D, Halloy José (2002) Robustness of circadian rhythms with respect to molecular noise. Proc Natl Acad Sci 99(2):673–678
Gonze D, Halloy J, Leloup JC, Goldbeter A (2003) Stochastic models for circadian rhythmes: effect of molecular noise on periodic and chaotic behaviour. C R Biol 326:189–203
Haschke R, Steil JJ (2005) Input-space bifurcation manifolds of recurrent neural networks. Neurocomputing 64:25–38
Lysetskiy M, Zurada JM (2004) Bifurcating neuron: computation and learning. Neural Netw 17:225–232
Nagy B (2008) Comparison of the bifurcation curves of a two-variable and a three-variable circadian rhythm model. Appl Math Model 38:1587–1598
Laroche B, Claude D (2004) Flatness-based control of PER protein oscillations in a Drosophila model. IEEE Trans Autom Control 49(2):175–183
Leloup JC, Gonze D, Goldbeter A (1999) Limit cycle models for circadian rhythms based on transcriptional regulation in Drosophila and Neurospora. J Biol Rhythms 14(6):433–448
Leloup JC, Gonze D, Goldbeter A (2006) Computational models for circadian rythms: deterministic versus stochastic approaches. Computational Systems Biology. Elsevier, Amsterdam
Lee JH, Sancar A (2011) Circadian clock disruption improves the efficacy of chemotherapy through p73-mediated apoptosis. In: Proceedings of the National Academy of Sciences
Lévi FA (2008) The circadian timing system: a coordinator of life processes. IEEE Eng Med Biol Mag 27(1):17–19
Liao X, Wong KW, Wu Z (2001) Bifurcation analysis of a two-neuron system with distributed delays. Phys D 149:123–141
Nagy B (2009) Analysis of the biological clock of Neurospora. J Comput Appl Math 226:298–305
Nguyen LH, Hong KS (2012) Hopf bifurcation control via a dynamic state-feedback control. Phys Lett A 376:442–446
Nguyen LH, Hong KS, Park S (2012) Bifurcation control of the Morris–Lecar neuron model via a dynamic state feedback ontrol. Biol Cybern 106:587–594
Rigatos GG, Siano P (2011) Design of robust electric power system stabilizers using Kharitonov’s theorem. Math Comput Simul 82(1):181–191
Rigatos G, Rigatou E (2013) A Kalman Filtering approach to robust synchronization of coupled neural oscillators. In: ICNAAM 2013, 11th international conference on numerical analysis and applied mathematics, Rhodes, Greece, Sep. 2013
Rigatos GG (2013) Advanced models of neural networks: nonlinear dynamics and stochasticity in biological neurons. Springer, Berlin
Song Y, Han M, Wei J (2005) Stability and Hopf bifurcation analysis on a simplified BAM network with delays. Phys D 200:185–204
That LT, Ding Z (2012) Reduced-order observer design of multi-output nonlinear systems with application to a circadian model. Trans Inst Meas Control 35(4):417–425
Toscano R, Lyonnet P (2010) Robust static output feedback controller synthesis using Kharitonov’s theorem and evolutionary algorithms. Inf Sci 180:20232028
Tsumoto K, Yoshinaga T, Iida H, Kawakami H, Ashara K (2006) Bifurcations in a mathematical model for circadian oscillations of clock genes. J Theor Biol 239:101–122
Vidal A, Zhang Q, Médigue C, Fabre S, Clément F (2012) DynPeak: An Algorithm for Pulse Detection and Frequency Analysis in Hormonal Time Series. PLoS One 7(7):e39001
Wang J, Chen L, Fei X (2004) Bifurcation control of the Hodgkin-Huxley equations. Chaos, Solitons and Fractals 33:217–224
Wang H, Yu Y, Zhu R, Wang S (2013) Two-parameter bifurcation in a two-dimensional simplified Hodgkin–Huxley model. Commun Nonlinear Sci Numer Simul 18:184–193
Xu X, Hu HY, Wang HL (2006) Stability switches, Hopf bifurcations and chaos of a neuron model with delay dependent parameters. Phys Lett A 354:126–136
Zhang J, Bierman A, Wen JT, Julius A, Figueiro M (2010) Circadian system modeling and phase control. In: 49th IEEE conference on decision and control, Dec. 2010
Zhang T, Wang J, Fei X, Deng B (2007) Synchronization of coupled FitzHugh–Nagumo systems via MIMO feedback linearization control. Chaos Solitons and Fractals 33(1):194–202
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix
A Stability analysis through the Routh–Hurwitz criterion
Since the Routh–Hurwitz criterion is meanwhile of the category well known but not widely known, a quick look at the ideas will be taken behind this classical method and a few concrete examples will be analyzed as illustrations of how to apply it.
The Routh–Hurwitz criterion provides a systematic method for finding out if all the roots of a polynomial lie in the left complex semi-plane. In the case of a dynamical system described in the s-frequency domain by a transfer function, one can find if the denominator’s characteristic polynomial contains only stable roots thus concluding that the system is also stable. Moreover, one can find out the number of complex eigenvalues which lie in the right complex semi-plane and thus are unstable. Additionally, one can find if there are roots of the polynomial with purely imaginary part, resulting a sustained oscillations dynamics (limit cycles).
1.1 The Routh–Hurwitz stability criterion
To evaluate the stability of the roots of the characteristic polynomial
the following Routh table is considered:
where
etc.
The Routh–Hurwitz stability analysis points out that the number of unstable roots of the characteristic polynomial (roots with positive real part) is equal to the number of changes of sign appearing in the first column of the Routh table.
Example 1
The characteristic polynomial \(p(s)=s^4+5s^3+20s^2+30s+40\) is considered. The associated Routh table is
No change of sign is observed in the first column of the Routh table. Therefore, the characteristic polynomial has no roots in the right complex semi-plane and is a stable one.
Example 2
The characteristic polynomial \(p(s)=s^3+2s^2+4s+30\) is considered. The associated Routh table is
There are two changes if sign in the first column of the Routh table; therefore, the characteristic polynomial has two unstable roots.
Example 3
The characteristic polynomial \(p(s)=s^5+2s^4+3s^3+6s^2+12s+18\) is considered. In this case, an element in the first column of the Routh table becomes equal to 0. This is substituted by a small positive number \(\epsilon \). Next, the sign of the elements of the first column of the Routh table is examined while \(\epsilon {\rightarrow }0\). Thus, the associated Routh table becomes
For \({\epsilon }{\rightarrow }0^{+}\), the elements of the first column become \(1,2,\epsilon >0,6-\frac{6}{\epsilon }<0,3,18\). There are two changes of sign in the elements of the first column of Routh table, and consequently, there are two unstable roots in the right complex semi-plane.
Example 4
The characteristic polynomial \(p(s)=s^5+6s^4+10s^3+35s^2+24s+44\) is considered. In such a case, complete row of the associated Routh table can become equal to zero. This happens when among the roots of the characteristic polynomial there are conjugate imaginary ones or complex roots of opposite sign. To process this case, an auxiliary characteristic polynomial is generated using the row that precedes the row where zeroing appeared. By dividing the initial characteristic polynomial with the auxiliary characteristic polynomial, one obtains a new characteristic polynomial for which the computation of the Routh table is repeated.
The Routh table of the initial characteristic polynomial is
The auxiliary characteristic polynomial is \(p(s)=11s^2+44\) with purely imaginary roots \(s_{1,2}={\pm }j2\). The new characteristic polynomial is computed using \(q(s)=p(s)/a(s)=s^3+6s^2+6s+11\). For \(q(s)\), the Routh table is
For the new Routh table, there is no change of sign in its first column; thus, all roots of \(q(s)\) lie in the left complex semi-plane. Consequently, for the initial characteristic polynomial, it holds \(p(s)=(11s^2+44)q(s)\) which means that \(p(s)\) has two purely imaginary roots (Hopf bifurcations) while the rest of its roots are stable.
Example 5
Consider the characteristic polynomial \(p(s)=s^6+2s^5+9s^2+12s^3+43s^2+50s+75\). The associated Routh table is
There is zeroing of one row. The auxiliary characteristic polynomial is formulated using the row preceding to zeroing, that is \(a(s)=3s^4+18s^3+75=3(s^4+6s^2+25)\). By diving the initial characteristic polynomial with the auxiliary characteristic polynomial, the new characteristic polynomial is obtained. That is
The polynomial \(q_1(s)=s^4+6s^2+25\) has complex roots (every two of them are opposite to each other), that is \(\rho _1=1+j2\), \(\rho _2=1-j2\), \(\rho _3=-1+j2\) and \(\rho _4=-1-j2\). The polynomial \(q_2(s)=s^2+2s+3\) has the complex conjugate roots \(\rho _1=-1+j\sqrt{2}\) and \(\rho _2=-1-j\sqrt{2}\). From the previous analysis, it can be seen that the characteristic polynomial \(p(s)\) has roots in the right complex semi-plane and is unstable.
1.2 Use of the Routh–Hurwitz criterion for the computation of relative stability
By performing the change of variable \(s{\rightarrow }p-a\) in the characteristic polynomial, one can find the relative position of the roots of the characteristic polynomial with respect to a reference value. This means that one can conclude if the roots of the characteristic polynomial are located to the left of the reference eigenvalue \(a\) (they decay faster than \(a\)) or to the right of the reference eigenvalue (they decay slower than \(a\)).
The following characteristic polynomial is considered: \(p(s)=s^3+10.2{s^2}+21s+2\) and the associated Routh table becomes
The system has the following stable poles \(p_1=-7.39\), \(p_2=-2.70\), \(p_3=-0.10\). It is examined if the poles of the system decay faster than the eigenvalue, \(a=-0.2\). The change of variables \(s=p-0.2\) or \(p=s+0.2\) is considered. The new characteristic polynomial is \(q(s)=p^3+9.6{p^2}+16.32p-1.728\) and the associated Routh table becomes
It can be noticed that there is one change of sign in the first column of the Routh table; therefore, the characteristic polynomial has an eigenvalue which lies to the right of the reference eigenvalue \(a=-0.2\).
Rights and permissions
About this article
Cite this article
Rigatos, G.G. Fixed-point bifurcation analysis in biological models using interval polynomials theory. Biol Cybern 108, 365–380 (2014). https://doi.org/10.1007/s00422-014-0605-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-014-0605-7