Abstract
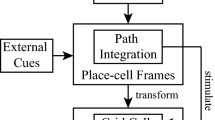
Inspired by recent biological experiments, we simulate animals moving in different environments (open space, spiral mazes and on a treadmill) to test the performances of a simple model of the retrosplenial cortex (RSC) acting as a path integration (PI) and as a categorization mechanism. The connection between the hippocampus, RSC and the entorhinal cortex is revealed through a novel perspective. We suppose that the path integration is performed by the information coming from RSC. Grid cells in the entorhinal cortex then can be built as the result of a modulo projection of RSC activity. In our model, PI is performed by a 1D field of neurons acting as a simple low-pass filter of head direction (HD) cells modulated by the linear velocity of the animal. Our paper focuses on the constraints on the HD cells shape for a good approximation of PI. Recording of neurons on our 1D PI field shows these neurons would not be intuitively interpreted as performing PI. Using inputs coming from a narrow neighbouring projection of our PI field creates place cell-like activities in the RSC when the mouse runs on the treadmill. This can be the result of local self-organizing maps representing blobs of neurons in the RSC (e.g. cortical columns). Other simulations show that accessing the whole PI field would induce place cells whatever the environment is. Since this property is not observed, we conclude that the categorization neurons in the RSC should have access to only a small fraction of the PI field.















Similar content being viewed by others
References
Alexander AS, Nitz DA (2015) Retrosplenial cortex maps the conjunction of internal and external spaces. Nat Neurosci 18(8):1143
Blayo F (1992) Kohonen self-organizing maps: is the normalization necessary? Complex Syst 6(6):105–123
Byrne P, Becker S, Burgess N (2007) Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol Rev 114(2):340
Collett T, Baron J, Sellen K (1996) On the encoding of movement vectors by honeybees. are distance and direction represented independently? J Comp Physiol A 179(3):395–406
Cooper BG, Mizumori SJ (1999) Retrosplenial cortex inactivation selectively impairs navigation in darkness. NeuroReport 10(3):625–630
Cooper BG, Manka TF, Mizumori SJ (2001) Finding your way in the dark: the retrosplenial cortex contributes to spatial memory and navigation without visual cues. Behav Neurosci 115(5):1012
Crawford ML, Harwerth RS, Smith EL, Mills S, Ewing B (2001) Experimental glaucoma in primates: changes in cytochrome oxidase blobs in v1 cortex. Investig Ophthalmol Visual Sci 42(2):358–364
Czajkowski R, Jayaprakash B, Wiltgen B, Rogerson T, Guzman-Karlsson MC, Barth AL, Trachtenberg JT, Silva AJ (2014) Encoding and storage of spatial information in the retrosplenial cortex. Proc Natl Acad Sci 111(23):8661–8666
Etienne AS, Jeffery KJ (2004) Path integration in mammals. Hippocampus 14(2):180–192
Fuhs MC, Touretzky DS (2006) A spin glass model of path integration in rat medial entorhinal cortex. J Neurosci 26(16):4266–4276
Gaussier P, Banquet J, Sargolini F, Giovannangeli C, Save E, Poucet B (2007) A model of grid cells involving extra hippocampal path integration, and the hippocampal loop. J Integr Neurosci 6(03):447–476
Gaussier P, Mingda J, Krichmar JL (2020) A joint paper submitted to scientific reports, a parsimonious computational model explaining path integration in cortex. Sci Rep (submitted)
Jacob P-Y, Casali G, Spieser L, Page H, Overington D, Jeffery K (2017) An independent, landmark-dominated head-direction signal in dysgranular retrosplenial cortex. Nat Neurosci 20(2):173
Jauffret A, Grand C, Cuperlier N, Gaussier P, Tarroux P (2013) How can a robot evaluate its own behavior? A neural model for self-assessment. In: The 2013 International Joint Conference on Neural Networks (IJCNN). IEEE, pp 1–8
Kohonen T (1990) The self-organizing map. Proc IEEE 78(9):1464–1480
Kropff E, Carmichael JE, Moser M-B, Moser EI (2015) Speed cells in the medial entorhinal cortex. Nature 523(7561):419
Lozano YR, Page H, Jacob P-Y, Lomi E, Street J, Jeffery K (2017) Retrosplenial and postsubicular head direction cells compared during visual landmark discrimination. Brain Neurosci Adv 1:2398212817721859
Mao D, Kandler S, McNaughton BL, Bonin V (2017) Sparse orthogonal population representation of spatial context in the retrosplenial cortex. Nat Commun 8(1):243
Markou M, Singh S (2003) Novelty detection: a review—part 2: neural network based approaches. Sig Process 83(12):2499–2521
McNaughton B, Chen L, Markus E (1991) “Dead reckoning,” landmark learning, and the sense of direction: a neurophysiological and computational hypothesis. J Cogn Neurosci 3(2):190–202
McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, Kudrimoti H, Qin Y, Skaggs W, Suster M et al (1996) Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J Exp Biol 199(1):173–185
McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser M-B (2006) Path integration and the neural basis of the’cognitive map’. Nat Rev Neurosci 7(8):663
Nitz DA (2012) Spaces within spaces: rat parietal cortex neurons register position across three reference frames. Nat Neurosci 15(10):1365
Poucet B, Chaillan F, Truchet B, Save E, Sargolini F, Hok V (2015) Is there a pilot in the brain? Contribution of the self-positioning system to spatial navigation. Front Behav Neurosci 9:292
Rall W (1960) Membrane potential transients and membrane time constant of motoneurons. Exp Neurol 2(5):503–532
Redish AD, Touretzky DS (1997) Cognitive maps beyond the hippocampus. Hippocampus 7(1):15–35
Rescorla RA, Wagner AR et al (1972) A theory of pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. Class Cond II Curr Res Theory 2:64–99
Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser M-B, Moser EI (2006) Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312(5774):758–762
Save E, Guazzelli A, Poucet B (2001) Dissociation of the effects of bilateral lesions of the dorsal hippocampus and parietal cortex on path integration in the rat. Behav Neurosci 115(6):1212
Sharp PE, Tinkelman A, Cho J (2001) Angular velocity and head direction signals recorded from the dorsal tegmental nucleus of gudden in the rat: implications for path integration in the head direction cell circuit. Behav Neurosci 115(3):571
Stone T, Webb B, Adden A, Weddig NB, Honkanen A, Templin R, Wcislo W, Scimeca L, Warrant E, Heinze S (2017) An anatomically constrained model for path integration in the bee brain. Curr Biol 27(20):3069–3085
Taube JS (1995) Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci 15(1):70–86
Taube JS (1998) Head direction cells and the neurophysiological basis for a sense of direction. Prog Neurobiol 55(3):225–256
Taube JS, Muller RU, Ranck JB (1990a) Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10(2):420–435
Taube JS, Muller RU, Ranck JB (1990b) Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci 10(2):436–447
Widrow B, Hoff ME (1960) Adaptive switching circuits. Technical report, Stanford Univ CA Stanford Electronics Labs
Zhang K (1996) Representation of spatial orientation by the intrinsic dynamics of the head-direction cell ensemble: a theory. J Neurosci 16(6):2112–2126
Acknowledgements
This work was supported by CY Cergy Paris University, CNRS scholar, Equipex Robotex and the Institute for Advanced Studies of CY university. We would like to show our gratitude to P.Y. Jacobs, Jeff Krichmar and D. Nitz for providing insight and expertise that assisted the research. We also thank Yuechen Li for comments that improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jean-Marc Fellous.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the special Issue entitled ‘Complex Spatial Navigation in Animals, Computational Models and Neuro-inspired Robots’.
Appendix
Appendix
1.1 Classical conditioning mechanism used as a STM
In the classical conditioning mechanism, \(dW_{ij} = W_{ij}(t+dt)-W_{ij}(t)\), we substitute \(dW_{ij}\) in Eq. (1). Thereby, the conditional output (vector field) \(O_i\) can be represented as:
\(O_i(t+dt)\) is inhibited to 0 when \(V_i(t-dt)=0\), otherwise,
when \(C_j=1\), \(O_i = C_j \cdot W_{ij} = W_{ij}\). Finally, the output can be rewritten as:
where \(\lambda \) is the learning rate altered between no stimulated rate 0.001 and strong stimulated rate 1 (when the animal meets the reward or the reset mechanism is triggered. When \(\lambda \) is small enough as the one we use for the simulation (\(\lambda \) = 0.001), the animal reserves the most of the state of the past PI and updates PI field step by step. While the reset is activated (\(\lambda \) = 1), the animal erases all the past PI and update to the current state immediately.
1.2 Kohonen map algorithm using scalar product
The activity of neurons on the Kohonen map \(S_k(t)\) is discretized from PI field. To cluster the activity on the map, we use the dot product to determine which pattern of weights \(W_{ik}(t)\) is the most similar to the vector of input activities \(P_i(t)\) (Fig.2). The number of the winner neuron \(k^w\) is defined by:
M is the number of neurons on a 1D Kohonen map. The weight between the neurons on PI field and on the Kohonen map is updated by:
\(\epsilon \) is the learning rate of self-organizing. With a neighbourhood function \(h_{kk^w}(t)\), we have the activity on the Kohonen map:
d is the coordinate distance between the winner neuron \(k^w\) and other neurons on a 1D Kohonen map. Figure 16 shows the shape of the neighbourhood function.
Rights and permissions
About this article
Cite this article
Ju, M., Gaussier, P. A model of path integration and representation of spatial context in the retrosplenial cortex. Biol Cybern 114, 303–313 (2020). https://doi.org/10.1007/s00422-020-00833-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-020-00833-x