Abstract
Neuroscience is of emerging importance along with the contributions of Operational Research to the practices of diagnosing neurodegenerative diseases with computer-aided systems based on brain image analysis. Although multiple biomarkers derived from Magnetic Resonance Imaging (MRI) data have proven to be effective in diagnosing Alzheimer’s disease (AD) and mild cognitive impairment (MCI), no specific system has yet been a part of routine clinical practice. This paper aims to introduce a fully-automated voxel-based procedure, Voxel-MARS, for detection of AD and MCI in early stages of progression. Performance was evaluated on a dataset of 508 MRI volumes gathered from the Alzheimer’s Disease Neuroimaging Initiative database. Data were transformed into a high-dimensional space through a feature extraction process. A novel 3-step feature selection procedure was applied. Multivariate Adaptive Regression Splines method was used as a classifier for the first time in the field of brain MRI analysis. The results were compared to those presented in a previous study on 28 voxel-based methods in terms of their ability to separate control normal (CN) subjects from the ones diagnosed with AD and MCI. It was observed that our method outperformed all of the others in sensitivity (83.58% in AD/CN and 78.38% in MCI/CN classification) with acceptable specificity values (over 85% in both cases). Furthermore, the method worked for discriminating MCI patients which converted to AD in 18 months (MCIc) from non-converters (MCInc) with a sensitivity outcome better than 27 of 28 methods. Overall, it was shown that the proposed method is promising in early detection of AD.








Similar content being viewed by others
Notes
A brain function syndrome which causes a slight decline in cognitive abilities and an increased risk of converting into AD.
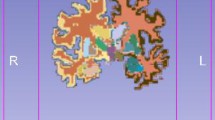
All 452 ICBM subject T1-weighted scans were aligned with the atlas space, corrected for scan inhomogeneities, and classified into gray matter, white matter, and cerebrospinal fluid. The 452 tissue maps were separated into their separate components and each component was averaged in atlas space across the subjects to create the probability fields for each tissue type. These fields represent the likelihood of finding gray matter, white matter, or cerebrospinal fluid at a specified position for a subject that has been linearly aligned to the atlas space (http://www.loni.usc.edu/atlases/Atlas_Methods.php?atlas_id=7).
Standard brain space defined by Montreal Neurological Institute (MNI).
The total number of the raw data features containing the probabilities for 3 tissue classes at each voxel is p. Therefore, considering only the gray matter tissue probabilities, the dimensionality is equal to the total number of voxels, which is (p / 3).
An explanatory example for the use of height threshold and extent threshold in fMRI analysis is provided in Friston et al. (1996).
Jekabsons G., ARESLab: Adaptive Regression Splines toolbox for MATLAB/Octave, 2011, available at http://www.cs.rtu.lv/jekabsons/.
The Matlab Toolbox for Dimensionality Reduction van der Maaten et al. (2009) was used for computations.
Here, the letters N and k are used with a tilde (\(\sim \)) sign over them in order not to be confused with the N used for expressing the sample size and the k appearing in Eq. (13) as the number of knots, respectively.
References
Adaszewski, S., Dukart, J., Kherif, F., Frackowiak, R., & Draganski, B. (2013). How early can we predict Alzheimer’s disease using computational anatomy? Neurobiology of Aging, 34(12), 2815–2826.
Alvarez, I., Gorriz, J., Ramirez, J., Salas-Gonzalez, D., Lopez, M., Puntonet, C., et al. (2009). Alzheimer’s diagnosis using eigenbrains and support vector machines. Electronics Letters, 45(7), 342–343.
Álvarez-Miranda, E., Farhan, H., Luipersbeck, M., & Sinnl, M. (2016). A bi-objective network design approach for discovering functional modules linking Golgi apparatus fragmentation and neuronal death. Annals of Operations Research, 1–26. http://link.springer.com/article/10.1007/s10479-016-2188-2.
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage, 38(1), 95–113.
Ashburner, J. (2009). Computational anatomy with the SPM software. Magnetic Resonance Imaging, 27(8), 1163–1174. (Proceedings of the international school on magnetic resonance and brain function).
Ashburner, J., & Friston, K. J. (2005). Unified segmentation. Neuroimage, 26(3), 839–851.
Boutet, C., Chupin, M., Lehricy, S., Marrakchi-Kacem, L., Epelbaum, S., Poupon, C., et al. (2014). Detection of volume loss in hippocampal layers in Alzheimer’s disease using 7T MRI: A feasibility study. Neuroimage: Clinical, 5, 341–348.
Chaves, R., Ramìrez, J., Górriz, J., López, M., Salas-Gonzalez, D., lvarez, I., et al. (2009). SVM-based computer-aided diagnosis of the Alzheimer’s disease using t-test NMSE feature selection with feature correlation weighting. Neuroscience Letters, 461(3), 293–297.
Chincarini, A., Bosco, P., Calvini, P., Gemme, G., Esposito, M., Olivieri, C., et al. (2011). Local MRI analysis approach in the diagnosis of early and prodromal Alzheimer’s disease. Neuroimage, 58(2), 469–480.
Chupin, M., Grardin, E., Cuingnet, R., Boutet, C., Lemieux, L., & Lehricy, S., et al. (2009a). Fully automatic hippocampus segmentation and classification in Alzheimer’s disease and mild cognitive impairment applied on data from ADNI. Hippocampus, 19(6), 579–587.
Chupin, M., Hammers, A., Liu, R., Colliot, O., Burdett, J., & Bardinet, E., et al. (2009b). Automatic segmentation of the hippocampus and the amygdala driven by hybrid constraints: Method and validation. Neuroimage, 46(3), 749–761.
Colliot, O., Chtelat, G., Chupin, M., Desgranges, B., Magnin, B., Benali, H., et al. (2008). Discrimination between Alzheimer disease, mild cognitive impairment, and normal aging by using automated segmentation of the hippocampus. Radiology, 248(1), 194–201.
Cuingnet, R., Gerardin, E., Tessieras, J., Auzias, G., Lehricy, S., Habert, M. O., et al. (2011). Automatic classification of patients with Alzheimer’s disease from structural MRI: A comparison of ten methods using the ADNI database. Neuroimage, 56(2), 766–781.
Davatzikos, C., Fan, Y., Wu, X., Shen, D., & Resnick, S. M. (2008). Detection of prodromal Alzheimer’s disease via pattern classification of magnetic resonance imaging. Neurobiology of Aging, 29(4), 514–523.
Frackowiak, R., Friston, K., Frith, C., Dolan, R., Price, C., Zeki, S., et al. (2003). Human Brain Function (2nd ed.). Cambridge: Academic Press.
Francis, L. (2003). Martian chronicles: Is MARS better than neural networks? In: Casualty Actuarial Society Forum (pp. 75–102).
Friedman, J. H. (1991). Multivariate adaptive regression splines. The Annals of Statistics, 19(1), 1–67.
Friston, K., Holmes, A., Poline, J. B., Price, C., & Frith, C. (1996). Detecting activations in PET and fMRI: Levels of inference and power. Neuroimage, 4(3), 223–235.
Gerardin, E., Chtelat, G., Chupin, M., Cuingnet, R., Desgranges, B., Kim, H. S., et al. (2009). Multidimensional classification of hippocampal shape features discriminates Alzheimer’s disease and mild cognitive impairment from normal aging. Neuroimage, 47(4), 1476–1486.
Graa, M., Termenon, M., Savio, A., Gonzalez-Pinto, A., Echeveste, J., Prez, J., et al. (2011). Computer aided diagnosis system for Alzheimer disease using brain diffusion tensor imaging features selected by Pearson’s correlation. Neuroscience Letters, 502(3), 225–229.
Hastie, T., Tibshirani, R., & Friedman, J. (2009). The elements of statistical learning: Data mining, inference and prediction (2nd ed.). Berlin: Springer.
Jack, C. R., Bernstein, M. A., Fox, N. C., Thompson, P., Alexander, G., Harvey, D., et al. (2008). The Alzheimer’s disease neuroimaging initiative (ADNI): MRI methods. Journal of Magnetic Resonance Imaging, 27(4), 685–691.
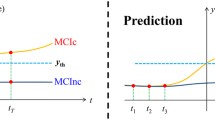
Jack, C. R., Knopman, D. S., Jagust, W. J., Shaw, L. M., Aisen, P. S., Weiner, M. W., et al. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet Neurology, 9(1), 119–128.
Jain, A., Duin, R. P. W., & Mao, J. (2000). Statistical pattern recognition: A review. IEEE Transactions on Pattern Analysis and Machine Intelligence, 22(1), 4–37.
Klöppel, S., Stonnington, C. M., Chu, C., Draganski, B., Scahill, R. I., Rohrer, J. D., et al. (2008). Automatic classification of MR scans in Alzheimer’s disease. Brain, 131(3), 681–689.
Li, M., Qin, Y., Gao, F., Zhu, W., & He, X. (2014). Discriminative analysis of multivariate features from structural MRI and diffusion tensor images. Magnetic Resonance Imaging, 32(8), 1043–1051.
Liu, S., Liu, S., Cai, W., Pujol ,S., Kikinis, R., & Feng, D. (2014). Early diagnosis of Alzheimer’s disease with deep learning. In: 2014 IEEE 11th International Symposium on Biomedical Imaging (ISBI) (pp. 1015–1018).
López, M., Ramìrez, J., Górriz, J., Álvarez, I., Salas-Gonzalez, D., Segovia, F., et al. (2011). Principal component analysis-based techniques and supervised classification schemes for the early detection of Alzheimer’s disease. Neurocomputing, 74(8), 1260–1271. (Selected papers from the 3rd international work-conference on the interplay between natural and artificial computation (IWINAC 2009)).
Magnin, B., Mesrob, L., Kinkingnhun, S., Plgrini-Issac, M., Colliot, O., Sarazin, M., et al. (2009). Support vector machine-based classification of Alzheimers disease from whole-brain anatomical MRI. Neuroradiology, 51(2), 73–83.
Misra, C., Fan, Y., & Davatzikos, C. (2009). Baseline and longitudinal patterns of brain atrophy in MCI patients, and their use in prediction of short-term conversion to AD: Results from ADNI. Neuroimage, 44(4), 1415–1422.
Moisen, G. G., & Frescino, T. S. (2002). Comparing five modelling techniques for predicting forest characteristics. Ecological Modelling, 157(23), 209–225.
Morra, J., Tu, Z., Apostolova, L., Green, A., Toga, A., & Thompson, P. (2010). Comparison of adaboost and support vector machines for detecting Alzheimer’s disease through automated hippocampal segmentation. IEEE Transactions on Medical Imaging, 29(1), 30–43.
Mwangi, B., Tian, T., & Soares, J. (2014). A review of feature reduction techniques in neuroimaging. Neuroinformatics, 12(2), 229–244.
Otsu, N. (1975). A threshold selection method from gray-level histograms. Automatica, 11(285–296), 23–27.
Özmen, A., Weber, G. W., Batmaz, ì, & Kropat, E. (2011). RCMARS: Robustification of cmars with different scenarios under polyhedral uncertainty set. Communications in Nonlinear Science and Numerical Simulation, 16(12), 4780–4787. (sI:Complex Systems and Chaos with Fractionality, Discontinuity, and Nonlinearity).
Padilla, P., Gorriz, J., Ramirez, J., Chaves, R., Segovia, F., Alvarez, I., et al. (2010). Alzheimer’s disease detection in functional images using 2D Gabor wavelet analysis. Electronics Letters, 46(8), 556–558.
Padilla, P., Lopez, M., Gorriz, J., Ramirez, J., Salas-Gonzalez, D., & Alvarez, I. (2012). NMF-SVM based cad tool applied to functional brain images for the diagnosis of Alzheimer’s disease. IEEE Transactions on Medical Imaging, 31(2), 207–216.
Park, H., Yang, J., Seo, J., & Lee, J. (2012). Dimensionality reduced cortical features and their use in the classification of Alzheimer’s disease and mild cognitive impairment. Neuroscience Letters, 529(2), 123–127.
Ramìrez, J., Górriz, J., Salas-Gonzalez, D., Romero, A., López, M., lvarez, I., et al. (2013). Computer-aided diagnosis of Alzheimers type dementia combining support vector machines and discriminant set of features. Information Sciences, 237, 59–72.
Ramìrez, J., Górriz, J., Segovia, F., Chaves, R., Salas-Gonzalez, D., López, M., et al. (2010). Computer aided diagnosis system for the Alzheimer’s disease based on partial least squares and random forest SPECT image classification. Neuroscience Letters, 472(2), 99–103.
Salas-Gonzalez, D., Górriz, J. M., Ramìrez, J., Illn, I. A., López, M., Segovia, F., Chaves, R., Padilla, P., Puntonet, C. G., & Alzheimers Disease Neuroimage Initiative, T. (2010). Feature selection using factor analysis for Alzheimers diagnosis using F18-FDG PET images. Medical Physics, 37(11), 6084–6095.
Salas-Gonzalez, D., Górriz, J. M., Ramìrez, J., López, M., Illan, I. A., Segovia, F., et al. (2009). Analysis of SPECT brain images for the diagnosis of Alzheimer’s disease using moments and support vector machines. Neuroscience Letters, 461(1), 60–64.
Savio, A., & Graa, M. (2013). Deformation based feature selection for computer aided diagnosis of Alzheimers disease. Expert Systems with Applications, 40(5), 1619–1628.
Segovia, F., Górriz, J., Ramìrez, J., Salas-Gonzalez, D., lvarez, I., López, M., et al. (2012). A comparative study of feature extraction methods for the diagnosis of Alzheimer’s disease using the ADNI database. Neurocomputing, 75(1), 64–71.
Shih, D. T., Kim, S. B., Chen, V. C. P., Rosenberger, J. M., & Pilla, V. L. (2014). Efficient computer experiment-based optimization through variable selection. Annals of Operations Research, 216(1), 287–305.
Strickland, J. (2014). Predictive modeling and analytics. LULU Press. https://books.google.com.tr/books?id=1jfXoQEACAAJ.
Suk, H. I., Lee, S. W., Shen, D., Initiative, A. D. N., et al. (2014). Hierarchical feature representation and multimodal fusion with deep learning for AD/MCI diagnosis. Neuroimage, 101, 569–582.
Tiraboschi, P., Hansen, L. A., Thal, L. J., & Corey-Bloom, J. (2004). The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology, 62(11), 1984–1989.
van der Maaten, L. J., Postma, E. O., & van den Herik, H. J. (2009). Dimensionality reduction: A comparative review. Journal of Machine Learning Research, 10(1–41), 66–71.
Vemuri, P., Gunter, J. L., Senjem, M. L., Whitwell, J. L., Kantarci, K., Knopman, D. S., et al. (2008). Alzheimer’s disease diagnosis in individual subjects using structural MR images: Validation studies. Neuroimage, 39(3), 1186–1197.
Weber, G. W., Batmaz, I., Köksal, G., Taylan, P., & Yerlikaya-Özkurt, F. (2012). CMARS: A new contribution to nonparametric regression with multivariate adaptive regression splines supported by continuous optimization. Inverse Problems in Science and Engineering, 20(3), 371–400.
Wendy, L., & Martinez, A. R. M. (2002). Computational statistics handbook with MATLAB. London: Chapman and Hall, CRC.
Westman, E., Simmons, A., Zhang, Y., Muehlboeck, J. S., Tunnard, C., Liu, Y., et al. (2011). Multivariate analysis of MRI data for Alzheimer’s disease, mild cognitive impairment and healthy controls. Neuroimage, 54(2), 1178–1187.
Yao, P. (2009). Hybrid fuzzy SVM model using CART and MARS for credit scoring. In Intelligent Human-Machine Systems and Cybernetics, 2009. IHMSC ’09. International Conference on (Vol. 2, pp. 392–395).
Ye, J., Chen, K., Wu, T., Li, J., Zhao, Z., Patel, R., Bae, M., Janardan, R., Liu, H., Alexander, G., & Reiman, E. (2008). Heterogeneous data fusion for Alzheimer’s disease study. In Proceedings of the 14th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining (pp. 1025–1033). ACM: New York, NY, USA, KDD’08.
Ye, J., Wu, T., Li, J., & Chen, K. (2011). Machine learning approaches for the neuroimaging study of Alzheimer’s disease. Computer, 44(4), 99–101.
Zhang, T., & Davatzikos, C. (2011). ODVBA: Optimally-discriminative voxel-based analysis. IEEE Transactions on Medical Imaging, 30(8), 1441–1454.
Zhang, W., & Goh, A. T. (2016). Multivariate adaptive regression splines and neural network models for prediction of pile drivability. Geoscience Frontiers, 7(1), 45–52.
Zhang, D., Wang, Y., Zhou, L., Yuan, H., & Shen, D. (2011). Multimodal classification of Alzheimer’s disease and mild cognitive impairment. Neuroimage, 55(3), 856–867.
Acknowledgements
This study is based on Alper Çevik’s Ph.D. thesis. B. Murat Eyüboğlu and Gerhard-Wilhelm Weber are the thesis co-supervisors. Kader Karlı Oğuz is a member of the thesis committee. This project has been supported by the Graduate School of Natural and Applied Sciences, METU Scientific Research Fund ‘BAP 07-02-2012-101’. The authors would like to thank Dr. Güçlü Ongun, Dr. Ayşe Özmen, and Dr. Semih Kuter for their valuable comments and suggestions to improve the study. We are also greatly indebted to Ajdan Küçükçiftçi for her proofreading which improved composition of this paper. Data collection and sharing for this study was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimers Association; Alzheimers Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.
Rights and permissions
About this article
Cite this article
Çevik, A., Weber, GW., Eyüboğlu, B.M. et al. Voxel-MARS: a method for early detection of Alzheimer’s disease by classification of structural brain MRI. Ann Oper Res 258, 31–57 (2017). https://doi.org/10.1007/s10479-017-2405-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10479-017-2405-7