Abstract
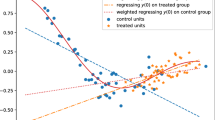
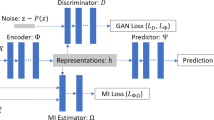
One fundamental problem in causal inference is the treatment effect estimation in observational studies, and its key challenge is to handle the confounding bias induced by the associations between covariates and treatment variable. In this paper, we study the problem of effect estimation on continuous treatment from observational data, going beyond previous work on binary treatments. Previous work on binary treatment focuses on de-confounding by balancing the distribution of covariates between the treated and control groups with either propensity score or confounder balancing techniques. In the continuous setting, those methods would fail as we can hardly evaluate the distribution of covariates under each treatment status. To tackle the case of continuous treatments, we propose a novel Generative Adversarial De-confounding (GAD) algorithm to eliminate the associations between covariates and treatment variable with two main steps: (1) generating an “calibration” distribution without associations between covariates and treatment by randomly perturbation on treatment variable; (2) learning sample weights that transfer the distribution of observed data to the “calibration” distribution for de-confounding with a Generative Adversarial Network. We show, both theoretically and with empirical experiments, that our GAD algorithm can remove the associations between covariates and treatment, hence, precisely estimating the causal effect of continuous treatment. Extensive experiments on both synthetic and real-world datasets demonstrate that our algorithm outperforms the state-of-the-art methods for effect estimation of continuous treatment with observational data.



Similar content being viewed by others
Notes
Units represent the objects of treatment. For example, in medical experiments, the units refer to the patients who take a particular medication.
\({\mathbf {X}}'\) should have the identical marginal distribution with the observed covariates, that is \(P({\mathbf {X}}') = P({\mathbf {X}})\).
References
Arjovsky M, Chintala S, Bottou L (2017) Wasserstein generative adversarial networks. In: Proceedings of the 34th international conference on machine learning, PMLR, proceedings of machine learning research, vol 70, pp 214–223
Athey S, Imbens G (2016) Recursive partitioning for heterogeneous causal effects. Proc Natl Acad Sci 113:7353–7360
Athey S, Imbens GW, Wager S (2018) Approximate residual balancing: debiased inference of average treatment effects in high dimensions. J R Stat Soc: Ser B (Stat Methodol) 80(4):597–623
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 46(3):399–424
Bang H, Robins JM (2005) Doubly robust estimation in missing data and causal inference models. Biometrics 61(4):962–973
Chan D, Ge R, Gershony O, Hesterberg T, Lambert D (2010) Evaluating online ad campaigns in a pipeline: causal models at scale. In: Proceedings of the 16th ACM SIGKDD international conference on Knowledge discovery and data mining, pp 7–16
Chan KG, Yam SC, Zhang Z (2016) Globally efficient non-parametric inference of average treatment effects by empirical balancing calibration weighting. J R Stat Soc Ser B Stat Methodol 78(3):673–700
Chernozhukov V, Chetverikov D, Demirer M, Duflo E, Hansen C et al (2016) Double machine learning for treatment and causal parameters. arXiv preprint arXiv:1608.00060
Duchi J, Namkoong H (2018) Learning models with uniform performance via distributionally robust optimization. arXiv preprint arXiv:1810.08750
Egel D, Graham BS, de Xavier Pinto CC (2008) Inverse probability tilting for moment condition models with missing data. Single equation models eJournal, Econometrics
Fan J, Imai K, Liu H, Ning Y, Yang X (2016) Improving covariate balancing propensity score: a doubly robust and efficient approach. Technical report
Flores CA, Flores-Lagunes A (2009) Identification and estimation of causal mechanisms and net effects of a treatment under unconfoundedness. IZA Institute of Labor Economics Discussion Paper Series
Fong C, Hazlett C, Imai K et al (2018) Covariate balancing propensity score for a continuous treatment: application to the efficacy of political advertisements. Ann Appl Stat 12(1):156–177
Galagate D (2016) Causal inference with a continuous treatment and outcome: alternative estimators for parametric dose-response functions with applications. Ph.D. thesis
Galvao AF, Wang L (2015) Uniformly semiparametric efficient estimation of treatment effects with a continuous treatment. J Am Stat Assoc 110(512):1528–1542
Goodfellow I, Pouget-Abadie J, Mirza M, Xu B, Warde-Farley D, Ozair S, Courville A, Bengio Y (2014) Generative adversarial nets. In: Advances in neural information processing systems, pp 2672–2680
Hainmueller J (2012) Entropy balancing for causal effects: a multivariate reweighting method to produce balanced samples in observational studies. Polit Anal 20(1):25–46
Hill JL (2011) Bayesian nonparametric modeling for causal inference. J Comput Graph Stat 20(1):217–240
Hirano K, Imbens GW (2004) The propensity score with continuous treatments. Applied Bayesian modeling and causal inference from incomplete-data perspectives 226164:73–84
Holland PW (1986) Statistics and causal inference. J Am Stat Assoc 81(396):945–960
Imai K, Ratkovic M (2014) Covariate balancing propensity score. J R Stat Soc: Ser B (Stat Methodol) 76(1):243–263
Imai K, Van Dyk DA (2004) Causal inference with general treatment regimes: generalizing the propensity score. J Am Stat Assoc 99(467):854–866
Imbens GW (2004) Nonparametric estimation of average treatment effects under exogeneity: a review. Rev Econ Stat 86(1):4–29
Imbens GW, Rubin DB (2015) Causal inference in statistics, social, and biomedical sciences. Cambridge University Press, Cambridge
Kallus N (2019) Generalized optimal matching methods for causal inference. J Mach Learn Res (forthcoming)
Kallus N, Santacatterina M (2019) Kernel optimal orthogonality weighting: a balancing approach to estimating effects of continuous treatments. arXiv, Methodology
Kallus N, Zhou A (2018) Policy evaluation and optimization with continuous treatments. In: International conference on artificial intelligence and statistics, pp 1243–1251
Kennedy EH, Ma Z, McHugh MD, Small DS (2017) Non-parametric methods for doubly robust estimation of continuous treatment effects. J R Stat Soc: Ser B (Stat Methodol) 79(4):1229–1245
Kohavi R, Longbotham R (2011) Unexpected results in online controlled experiments. ACM SIGKDD Explor Newsl 12(2):31–35
Kreif N, Grieve R, Díaz I, Harrison D (2015) Evaluation of the effect of a continuous treatment: a machine learning approach with an application to treatment for traumatic brain injury. Health Econ 24(9):1213–1228
Kuang K, Cui P, Li B, Jiang M, Yang S (2017) Estimating treatment effect in the wild via differentiated confounder balancing. In: Proceedings of the 23rd ACM SIGKDD international conference on knowledge discovery and data mining, pp 265–274. ACM
Kuang K, Cui P, Athey S, Xiong R, Li B (2018) Stable prediction across unknown environments. In: Proceedings of the 24th ACM SIGKDD international conference on knowledge discovery & data mining, pp 1617–1626
Kuang K, Cui P, Li B, Jiang M, Wang Y, Wu F, Yang S (2019) Treatment effect estimation via differentiated confounder balancing and regression. ACM Trans Knowl Discov Data (TKDD) 14(1):1–25
Kuang K, Cui P, Zou H, Li B, Tao J, Wu F, Yang S (2020) Data-driven variable decomposition for treatment effect estimation. IEEE Trans Knowl Data Eng. https://doi.org/10.1109/TKDE.2020.3006898
Kuang K, Li L, Geng Z, Xu L, Zhang K, Liao B, Huang H, Ding P, Miao W, Jiang Z (2020b) Causal inference. Engineering 6(3):253–263
Künzel SR, Sekhon JS, Bickel PJ, Yu B (2019) Metalearners for estimating heterogeneous treatment effects using machine learning. Proc Natl Acad Sci 116(10):4156–4165
Li F, Li L, Yin J, Zhang Y, Zhou Q, Kuang K (2020a) How to interpret machine knowledge. Engineering 6(3):218–220
Li M, Kuang K, Zhu Q, Chen X, Guo Q, Wu F (2020b) IB-M: a flexible framework to align an interpretable model and a black-box model. In: 2020 IEEE international conference on bioinformatics and biomedicine (BIBM), pp 643–649. IEEE
Liu J, Ma Y, Wang L (2018) An alternative robust estimator of average treatment effect in causal inference. Biometrics 74(3):910–923
Liu Y, Dieng A, Roy S, Rudin C, Volfovsky A (2019) Interpretable almost matching exactly for causal inference. AISTATS
Louizos C, Shalit U, Mooij J, Sontag D, Zemel R, Welling M (2017) Causal effect inference with deep latent-variable models. In: Proceedings of the 31st annual conference on neural information processing systems
Lu C, Wang S (2020) The general-purpose intelligent agent. Engineering 6(3):221–226
McCaffrey DF, Ridgeway G, Morral AR (2004) Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods 9(4):403
Neugebauer R, van der Laan M (2007) Nonparametric causal effects based on marginal structural models. J Stat Plan Inference 137(2):419–434
Olaya D, Coussement K, Verbeke W (2020) A survey and benchmarking study of multitreatment uplift modeling. Data Min Knowl Disc 34:273–308
Pearl J (2009) Causality. Cambridge University Press, Cambridge
Ren K, Zheng T, Qin Z, Liu X (2020) Adversarial attacks and defenses in deep learning. Engineering 6(3):346–360
Robins J, Rotnitzky A (2001) Comment on inference for semiparametric models: some questions and an answer, by P.J. Bickel and J. Kwon. Stat Sin 11:920–936
Robins JM, Hernan MA, Brumback B (2000) Marginal structural models and causal inference in epidemiology
Rojas-Carulla M, Schölkopf B, Turner R, Peters J (2018) Invariant models for causal transfer learning. J Mach Learn Res 19(1):1309–1342
Rong G, Mendez A, Assi EB, Zhao B, Sawan M (2020) Artificial intelligence in healthcare: review and prediction case studies. Engineering 6(3):291–301
Rosenbaum PR, Rubin DB (1983) The central role of the propensity score in observational studies for causal effects. Biometrika 70(1):41–55
Rubin DB (1974) Estimating causal effects of treatments in randomized and nonrandomized studies. J Educ Psychol 66(5):688
Rudas K, Jaroszewicz S (2018) Linear regression for uplift modeling. Data Min Knowl Disc 32:1275–1305
Rudin C (2019) Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat Mach Intell 1(5):206–215
Schölkopf B, Locatello F, Bauer S, Ke NR, Kalchbrenner N, Goyal A, Bengio Y (2021) Toward causal representation learning. Proc IEEE 109(5):612–634
Soltys M, Jaroszewicz S, Rzepakowski P (2014) Ensemble methods for uplift modeling. Data Min Knowl Disc 29:1531–1559
Tan Z (2010) Bounded, efficient and doubly robust estimation with inverse weighting. Biometrika 97:661–682
Tian Q, Kuang K, Jiang K, Wu F, Wang Y (2021) Analysis and applications of class-wise robustness in adversarial training. arXiv preprint arXiv:2105.14240
Wager S, Athey S (2015) Estimation and inference of heterogeneous treatment effects using random forests. J Am Stat Assoc 113:1228–1242
Westreich D, Lessler J, Funk MJ (2010) Propensity score estimation: neural networks, support vector machines, decision trees (cart), and meta-classifiers as alternatives to logistic regression. J Clin Epidemiol 63(8):826–833
Zhao Q (2016) Covariate balancing propensity score by tailored loss functions. arXiv, Methodology
Zhu Y, Coffman D, Ghosh D (2015) A boosting algorithm for estimating generalized propensity scores with continuous treatments. J Causal Inference 3:25–40
Zou WY, Shyam S, Mui M, Wang M, Pedersen J, Ghahramani Z (2020) Learning continuous treatment policy and bipartite embeddings for matching with heterogeneous causal effects. arXiv:2004.09703
Zubizarreta J (2015) Stable weights that balance covariates for estimation with incomplete outcome data. J Am Stat Assoc 110:910–922
Žliobaitė I (2017) Measuring discrimination in algorithmic decision making. Data Min Knowl Disc 31:1060–1089
Acknowledgements
This work was supported in part by National Natural Science Foundation of China (Nos. 61625107, 62006207), National Key Research and Development Program of China (Nos. 2018AAA0101900, 2020YFC0832500), the Fundamental Research Funds for the Central Universities and Zhejiang Province Natural Science Foundation (No. LQ21F020020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Sriraam Natarajan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuang, K., Li, Y., Li, B. et al. Continuous treatment effect estimation via generative adversarial de-confounding. Data Min Knowl Disc 35, 2467–2497 (2021). https://doi.org/10.1007/s10618-021-00797-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10618-021-00797-x