Summary
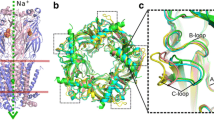
The N-terminal extracellular regions of heterooligomeric 3AB-type human 5-hydroxytryptamine receptors (5-HT 3ABR) were modelled based on the crystal structure of snail acetylcholine binding protein AChBP. Stepwise rotation of subunit A by 5° was performed between -10° and 15° to mimic agonist binding and receptor activation. Anticlockwise rotation reduced the size of the binding cavity in interface AB and reorganised the network of hydrogen bonds along the interface. AB subunit dimers with different rotations were applied for docking of ligands with different efficacies: 5-HT, m-chlorophenylbiguanide, SR 57227, quinolinyl piperazine and lerisetron derivatives. All ligands were docked into the dimer with −10° rotation representing ligand-free, open binding cavities similarly, without pharmacological discrimination. Their ammonium ions were in hydrogen bonding distance to the backbone carbonyl of W183. Anticlockwise rotation and contraction of the binding cavity led to distinctive docking interactions of agonists with E129 and cation–π interactions of their ammonium ions. Side chains of several further amino acids participating in docking (Y143, Y153, Y234 and E236) are in agreement with the effects of point mutations in the binding loops. Our model postulates that 5-HT binds to W183 in a hydrophobic cleft as well as to E236 in a hydrophilic vestibule. Then it elicits anticlockwise rotation to draw in loop C via π–cation–π interactions of␣its ammonium ion with W183 and Y234. Finally, closure of the binding cavity might end in rebinding of 5-HT to E129 in the hydrophilic vestibule.
Similar content being viewed by others
Abbreviations
- AChBP:
-
acetylcholine binding protein
- AMBER:
-
assisted model building with energy refinement
- AQPA:
-
arylquinolinyl piperazine
- BQPA:
-
bicyclooctano-quinolinyl piperazine
- CPBG:
-
m-chlorophenylbiguanide
- GABAA:
-
γ-aminobutyric acidA
- 5-HT:
-
5-hydroxytryptamine (serotonin)
- LGA:
-
Lamarckian genetic algorithm
- PDB:
-
protein data bank
- RMSD:
-
relative mean standard deviation
- SR 57227:
-
4-amino-(6-chloro-2-pyridyl)-1-piperidine HCl
- TM:
-
transmembrane.
References
R. Jin T.G. Banke M.L. Mayer S.F. Traynelis E. Gouaux (2003) Nat. Neurosci. 6 803
N. Unwin (1995) Nature 373 37
N. Unwin (2003) FEBS Lett. 555 91
M.O. Ortells G.G. Lunt (1995) Trends Neurosci. 18 121
A.J. Greenshaw P.H. Silverstone (1997) Drugs 53 20
F.G. Boess R. Beroukhim I.L. Martin (1995) J. Neurochem. 64 1401
M. Morales S.D. Wang (2002) J. Neurosci. 22 6732
A.M. Karnovsky L.F. Gotow D.D. McKinley J.L. Piechan C.L. Ruble C.J. Mills K.A.B. Schellin J.L. Slightom L.R. Fitzgerald C.W. Benjamin S.L. Roberts (2003) Gene 319 137
B. Niesler B. Frank J. Kapeller G.A. Rappold (2003) Gene 310 101
K. Brejc W. Van Dijk R.V. Klaassen M. Schuurmans J. VanDer Oost A.B. Smit T. Sixma (2001) Nature 411 269 Occurrence Handle10.1038/35077011 Occurrence Handle1:CAS:528:DC%2BD3MXjvF2qsbc%3D Occurrence Handle11357122
R.B. Russell M.A. Saqi R.A. Sayle P.A. Bates M.J.E. Sternberg (1997) J. Mol. Biol. 269 423
P.H.N. Celie S.E. Van Rossum-Fikkert W.J. Van Dijk K. Brejc A.B. Smit T.K. Sixma (2004) Neuron 41 907
T. Grutter J.P. Changeux (2001) Trends Biochem. Sci. 26 459
B.A. Cromer C.J. Morton M.W. Parker (2002) Trends Biochem. Sci. 27 280
B. Laube G. Maksay R. Schemm H. Betz (2002) Trends Pharmacol. Sci. 23 519
V. Costa A. Nistri A. Cavalli P. Carloni (2003) Br. J. Pharmacol. 140 921
D.S. Reeves M.F.R. Sayed P.L. Chau K.L. Price S.C.R. Lummis (2003) Biophys. J. 84 2338
G. Maksay Zs. Bikádi M. Simonyi (2003) J. Receptors Signal Transduct. 23 255
D.C. Reeves S.C.R. Lummis (2002) Mol. Membrane Biol. 19 11
P. Venkataraman S.P. Venkatachalan P.R. Joshi M. Muthalagi M.K. Schulte (2002) BMC Biochem. 3 15
D.L. Beene K.L. Price H.A. Lester D.A. Dougherty S.C.R. Lummis (2004) J. Neurosci. 24 9097
C. Schreiter R. Hovius M. Costioli H. Pick S. Kellenberger L. Schild H. Vogel (2003) J. Biol. Chem. 278 22709
X.Q. Hu L. Zhang R.R. Stewart F.F. Weight (2003) J. Biol. Chem. 278 46583
F.G. Boess L.J. Steward J.A. Steele D. Liu J. Reid T.A. Glencorse I.L. Martin (1997) Neuropharmacology 36 637
L.J. Steward F.G. Boess J.A. Steele D. Liu N. Wong I.L. Martin (2000) Mol. Pharmacol. 57 1249
P.A. Davies M. Pistis M.C. Hanna J.A. Peters J.L. Lambert T.G. Hales E.F. Kirkness (1999) Nature 397 359
C.H. Wu H. Huang L. Arminski J.C. Alvear Y. Chen Z.Z. Hu R.S. Ledley C. Lewis H.W. Mewes B.C. Orcutt B.E. Suzek A. Tsugita C.R. Vinayaga L.S.L. Yeh J. Zhang W.C. Barker (2002) Nucleic Acids Res. 30 35
J.D. Thompson D.G. Higgins T.J. Ibson (1994) Nucleic Acids Res. 22 4673 Occurrence Handle1:CAS:528:DyaK2MXitlSgu74%3D Occurrence Handle7984417
Z. Xiang B. Honig (2001) J. Mol. Biol. 311 421
Z. Xiang C. Soto B. Honig (2002) Proc. Natl. Acad. Sci. USA 99 7432
R.A. Laskowski M.W. MacArthur D.S. Moss J.M. Thornton (1993) J. Appl. Crystallogr. 26 283
I.K. McDonald J.M. Thornton (1994) J. Mol. Biol. 238 777
G.J. Kleywegt T.A. Jones (1994) Acta Crystallogr. D 50 178
M.L. Connolly (1983) Science 221 709
F. Melo E. Feytmans (1998) J. Mol. Biol. 277 1141
G.M. Morris D.S. Goodsell R.S. Hallaway R. Huey W.E. Hart R.K. Belew A.J. Olson (1998) J. Comput. Chem. 19 1639
W.D. Cornell P. Cieplak C.I. Bayly I.R. Gould K.M. Merz SuffixJr. D.M. Ferguson D.C. Spellmeyer T. Fox J.W. Caldwell P.A. Kollman (1995) J. Am. Chem. Soc. 117 5179
A. Bachy P.E. Keane H. Gozlan M. Hamon P. Delagne J. Lassalle P. Soubrié (1993) Br. J. Pharmacol. 108 256P
G.J. Kilpatrick A. Butler J. Burridge A.W. Oxford (1990) Eur. J. Pharmacol. 182 193
M.I. Sepúlveda S.C.R. Lummis I.L. Martin (1991) Br. J. Pharmacol. 104 536
H.S. Parihar A. Suryanarayanan C. Ma P. Joshi P. Venkataraman M.K. Schulte K.S. Kirschbaum (2001) Bioorg. Med. Chem. Lett. 11 2133
A. Cappelli M. Anzini S. Vomero L. Canullo L. Mennuni F. Makovec E. Doucet M. Hamon C. Menziani P.D. Benedetti G. Bruni M.R. Romero G. Giorgi A. Donati (1999) J. Med. Chem. 42 1556
P. Venkataraman P. Joshi S.P. Venkatachalan M. Muthalagi H.S. Parihar K.S. Kirschbaum M.K. Schulte (2002) BMC Biochem. 3 16
R.H. Henchman H.L. Wang S.M. Sine P. Taylor J.A. McCammon (2003) Biophys. J. 85 3007
Dubin, A.E., Erlander, M.G., Huvar, A., Huvar, R. and Buehler, L.K., US Patent No. 6,365,370, 1999.
D. Yan M.K. Schulte K.E. Bloom M.M. White (1999) J. Biol. Chem. 274 5537
A.D. Spier S.C.R. Lummis (2000) J. Biol. Chem. 275 5620
D.L. Beene G.S. Brandt W. Zhong N.M. Zacharias H.A. Lester D.A. Dougherty (2002) Biochemistry 41 10262
A.P. Tairi R. Hovius H. Pick H. Blasey A. Bernard A. Surprenant K. Lundström H. Vogel (1998) Biochemistry 37 15850
S. Lankiewicz N. Lobitz C.H.R. Wetzel R. Rupprecht G. Gisselmann H. Hatt (1998) Mol. Pharmacol. 53 202
S.B. Hansen Z. Radic T.T. Talley B.E. Molles T. Deerinck I. Tsigelny P. Taylor (2002) J. Biol. Chem. 277 41299
K.L. Price S.C.R. Lummis (2004) J. Biol. Chem. 279 23294
C. Bouzat F. Gumilar G. Spitzmaul H.L. Wang D. Rayes S.B. Hansen P. Taylor S.M. Sine (2004) Nature 430 896 Occurrence Handle10.1038/nature02753 Occurrence Handle1:CAS:528:DC%2BD2cXmslCnuro%3D Occurrence Handle15318223
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors with equal contribution to the manuscript.
Rights and permissions
About this article
Cite this article
Maksay, G., Simonyi, M. & Bikádi, Z. Subunit rotation models activation of serotonin 5-HT3AB receptors by agonists. J Comput Aided Mol Des 18, 651–664 (2004). https://doi.org/10.1007/s10822-004-6259-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-004-6259-0