Summary
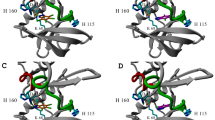
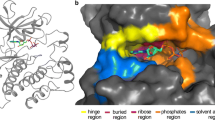
We have carried out up to 8.0 ns molecular dynamics simulation on the ATP-bound complexes of EGFR and HER-2 (homology model) receptor kinase domains to explore the possible consequences of amino acid residue changes in or close to the ATP site that might provide insights for selectivity of these kinases towards ATP site inhibitors. The simulation results show the formation of a channel under Thr766 following the movement of the side chain of Gln767 away from the hinge in EGFR. In HER-2, a similar movement of Gln799 occurs, but a simultaneous movement of Arg784 towards the hinge region occurs that tends to close the channel. The movement of Arg784 in HER-2 appears to result from the absence of an anchoring residue like Asp746 in EGFR, which has been changed to Gly778 in HER-2. In EGFR, this Arg784 is held away from the hinge region by interaction with Asp746, thereby leaving the channel open. This might be an important contributory factor to differences in selectivity of the ligands between the two kinases, probably more so than the conservative change of Cys751 of EGFR to serine in HER-2 at the ATP site.
Similar content being viewed by others
References
W.J. Fantl D.E. Johnson L.T. Williams (1993) Ann. Rev. Biochem. 62 453 Occurrence Handle7688944
A. Ullrich J. Schlessinger (1991) Cell 61 203 Occurrence Handle10.1016/0092-8674(90)90801-K
K.S. Kolibaba B.J. Druker (1997) Biochim. Biophys. Acta 1333 F217 Occurrence Handle9426205
S.A. Aaronson (1991) Science 254 1146 Occurrence Handle1659742
S. Menard S.M. Pupa M. Campiglio E. Tagliabue (2003) Oncogene 22 6570 Occurrence Handle10.1038/sj.onc.1206779 Occurrence Handle14528282
A.J. Bridges (2001) Chem. Rev. 101 2541 Occurrence Handle10.1021/cr000250y Occurrence Handle11749388
J. Stamos M.X. Sliwkowski C. Eigenbrot (2002) J. Biol. Chem. 277 46,265
S. Kamath J.K. Buolamwini (2003) J. Med. Chem. 46 4657 Occurrence Handle10.1021/jm030065n Occurrence Handle14561085
S. Blencke A. Ullrich H.J. Daub (2003) Biol. Chem. 278 15,435
G. Jones P. Willet R.C. Glen A.R. Leach R. Taylor (1997) J. Mol. Biol. 267 727 Occurrence Handle10.1006/jmbi.1996.0897 Occurrence Handle9126849
L. Kale R. Skeel M. Bhandarkar R. Brunner A. Gursoy N. Krawetz J. Phillips A. Shinozaki K. Varadarajan K. Schulten (1999) J. Comp. Phys. 151 283 Occurrence Handle10.1006/jcph.1999.6201
B.R. Brooks R.E. Bruccoleri B.D. Olafson D.J. States S. Swaminathan M. Karplus (1983) J. Comp. Chem. 4 187 Occurrence Handle10.1002/jcc.540040211
A.D. MacKerell SuffixJr. D. Bashford M. Bellott R.L. Dunbrack SuffixJr. J.D. Evanseck M.J. Field S. Fischer J. Gao H. Guo S. Ha D. Joseph-McCarthy L. Kuchnir K. Kuczera F.T.K. Lau C. Mattos S. Michnick T. Ngo D.T. Nguyen B. Prodhom W.E. Reiher SuffixIII B. Roux M. Schlenkrich J.C. Smith R. Stote J. Straub M. Watanabe J. Wiorkiewicz-Kuczera D. Yin M. Karplus (1998) J. Phys. Chem. B 102 3586 Occurrence Handle10.1021/jp973084f
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamath, S.A., Buolamwini, J.K. Asp746 to Glycine Change May have a Greater Influence than Cys751 to Serine Change in Accounting for Ligand Selectivity between EGFR and HER-2 at the ATP Site. J Comput Aided Mol Des 19, 287–291 (2005). https://doi.org/10.1007/s10822-005-5083-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10822-005-5083-5