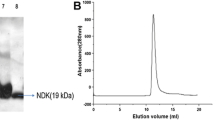
Abstract
Leishmania donovani dipeptidylcarboxypeptidsae (LdDCP), an angiotensin converting enzyme (ACE) related metallopeptidase has been identified and characterized as a putative drug target for antileishmanial chemotherapy. The kinetic parameters for LdDCP with substrate, Hip-His-Leu were determined as, Km, 4 mM and Vmax, 1.173 μmole/ml/min. Inhibition studies revealed that known ACE inhibitors (captopril and bradykinin potentiating peptide; BPP1) were weak inhibitors for LdDCP as compared to human testicular ACE (htACE) with Ki values of 35.8 nM and 3.9 μM, respectively. Three dimensional model of LdDCP was generated based on crystal structure of Escherichia coli DCP (EcDCP) by means of comparative modeling and assessed using PROSAII, PROCHECK and WHATIF. Captopril docking with htACE, LdDCP and EcDCP and analysis of molecular electrostatic potentials (MEP) suggested that the active site domain of three enzymes has several minor but potentially important structural differences. These differences could be exploited for designing selective inhibitor of LdDCP thereby antileishmanial compounds either by denovo drug design or virtual screening of small molecule databases.









Similar content being viewed by others
References
Handman E (2001) Leishmaniasis: current status of vaccine development. Clin Microbio Rev 14:229
Ashford RW, Desjeux P, Deraadt P (1992) Estimation of population at risk of infection and number of cases of Leishmaniasis. Parasitol Today 8:104
Molyneux D, Killick-Kendrick R (1987) Morphology, ultrastructure and lifecycles. In: The Leishmaniasis in biology and medicine, vol 1, pp 121
Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, Deseux P, Wasunna MK, Bryceson AD (2002) Visceral leishmaniasis: current status of control, diagnosis and treatment and a proposed research and development agenda. Lancet Infect Dis 2:494
Rijal S, Chappuis F, Singh R, Bovier PA, Achrya A, Karki BM, Das ML, Deseux P, Loutan L, Koirala S (2003) Treatment of visceral leishmaniasis in south eastern Nepal: decreasing efficacy of sodium stibogluconate and need for policy to limit further decline. Trans R Soc Trop Med Hyg 97:350
Goyal N, Duncan R, Selvapandiyan A, Debrabant A, Baig MS, Nakhasi HL (2006) Cloning and characterization of angiotensin converting enzyme related dipeptidylcarboxypeptidase from Leishmania donovani. Mol Biochem Parasitol 145:147
Fiser A (2004) Protein structure modeling in the proteomics era. Expert Rev Proteomics 1:97
Lesk AM, Chothia C (1980) How different amino acid sequences determine similar protein structures: the structure and evolutionary dynamics of the globins. J Mol Biol 136:225
Chothia C, Lesk AM (1986) The relation between the divergence of sequence and structure in proteins. EMBO J 5:823
Comellas-Bigler M, Lang R, Bode W, Maskos K (2005) Crystal structure of E. coli dipeptidylcarboxypeptidase DCP:future indication of a ligand dependent hinge movement mechanism. J Mol Biol 349:99
Lammeli UK (1970) Cleavage of structrural proteins during the assembly of the head of bacteriphage T4. Nature 227:80
Cushman DW, Cheung HS (1971) Spectrophotometric assay for angiotensin converting enzyme. Biochem Pharmacol 20:1637
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248
Sali A, Blundell TL (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234:779
Peng Y, Keenan SM, Welsh WJ (2005) Structural model of Plasmodium CDK, Pfmrk, a novel target for malaria chemotherapy. J Mol Graph Mod 24:72
Gellert A, Salanki K, NaraySzabo G (2006) Homology modeling and protein structure based functional analysis of cucumovirus coat proteins. J Mol Graph Mod 24:319
Heo J, Vaidehi N, Wendel William A (2007) Prediction of 3D structure of rat Mrg A G protein coupled receptor and identification of its binding site. J Mol Graph Mod 26:800
Laskowaski RA, McArther MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check sterio-chemical quality of a protein structures. J Appl Crystalogr 26:283
Hooft RWW, Vriend G, Sander C, Abola EE (1996) Errors in protein structures. Nature 381:272
Hooft RWW, Sander C, Vriend G (1996) Verification of protein structures: side chain planarity. J Appl Crystallog 29:714
Natesh R, Schwager SL, Sturrock ED, Acharya KR (2003) Crystal structure of the human angiotensin-converting enzyme-lisinopril complex. Nature 421:551
Nicholls A, Sharp KA, Honig B (1991) Protein Folding and Association: Insights from the Interfacial and Thermodynamic Properties of Hydrocarbons. Proteins Stuc Func Genet 11:281
Politzer P, Laurence PR, Jayasuriya K (1985) Molecular electrostatic potentials: an effective tool for the elucidation of biochemical phenomena. Environ Health Perspect 61:191
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J Comput Chem 25:1605
Mottram JC, Coombs GH, Alexander J (2004) Cysteine peptidase as virulence factors of Leishmania. Curr Opin Microbiol 7:375
Yao C, Donelson JE, Wilson ME (2003) The major surface protease (MSP or GP63) of Leishmania species: biosynthesis, regulation of expression and function. Mol Biochem Parasitol 132:1
Andrade Ribeirode AS, Santero MM, de Melo NN, Mares-Guia M (1998) Leishmania (Leishmania) amazonensis: purification and enzymatic characterization of soluble serein oligopeptidase. Exp Parasitol 89:153
Morty RE, Morehead J (2002) Cloning and characterization of leucylaminopeptidase from three pathogenic Leishmania species. J Biol Chem 277:26057
Yaron A, Mlynar D, Berger A (1972) A dipeptidocarboxypeptidase from E. coli. Biochem Biophys Res Commun 47:897
Soffer RL, Das M, Caldwell PR, Seegal BC, Hsu KC (1976) Biological and biochemical properties of angiotensin converting enzyme. Agents Actions 6:534
Lanzillo JJ, Dasarathy Y, Stevens J, Bardin CW, Fanburg BL (1985) Human testicular angiotensin-converting enzyme is a mixture of two molecular weight forms, Only one is similar to the seminal plasma enzyme. Biochem Biophys Res Commun 128:457
Henrich B, Becker S, Schroeder U, Plapp R (1993) dcp Gene of Escherichia coli: Cloning, sequencing, transcript mapping and characterization of the gene product. J Bacteriol 175:7290
Deddish PA, Wang LX, Jackman HL, Michel B, Wang J, Skidgel RA, Erdös EG (1996) J Pharmacol Exp Ther 279:1582
Deutch CE, Soffer RL (1978) Escherichia coli mutant defective in dipeptidyl carboxypeptidase. Pro Natl Acad Sci USA 75:5998
Junot C, Gonzales MF, Ezan E, Cotton J, Vazeux G, Michaud A, Azizi M, Vassiliou S, Yiotakis A, Corvol P, Dive V (2001) RXP 407, a selective inhibitor of the N-domain of angiotensin I-converting enzyme, blocks in vivo the degradation of hemoregulatory peptide acetyl-Ser-Asp-Lys-Pro with no effect on angiotensin I hydrolysis. J Pharmacol Exp Ther 297:606
Singh N, Chevé G, Avery MA, McCurdy CR (2006) Comparative protein modeling of 1-deoxy-D-xylulose-5-phosphate reductoisomerase enzyme from Plasmodium falciparum: a potential target for antimalarial drug discovery. J Chem Inf Model 46:1360
Sinha N, Smith-Gill SJ (2002) Electrostatics in protein binding and function. Curr Protein Pept Sci 3:601
Acknowledgments
This manuscript carries CDRI communication number 7523. The work is supported by grant from Council of Scientific and Industrial Research (CSIR) funded network project NPW0038 ‘Identification and validation of drug targets for selected pathogen’ and CSIR for financial support to M.S.B and Ashutosh Kumar.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mirza Saqib Baig and Ashutosh Kumar have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10822_2009_9315_MOESM2_ESM.tif
Ramachandran plot (LdDCP) of ¢/ψ distribution produced by PROCHECK validation package. The most favored and favored regions are colored red and dark yellow, respectively. Light yellow region are generally allowed, disallowed regions are white. (TIFF 4,203 kb)
Rights and permissions
About this article
Cite this article
Baig, M.S., Kumar, A., Siddiqi, M.I. et al. Characterization of dipeptidylcarboxypeptidase of Leishmania donovani: a molecular model for structure based design of antileishmanials. J Comput Aided Mol Des 24, 77–87 (2010). https://doi.org/10.1007/s10822-009-9315-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-009-9315-y