Abstract
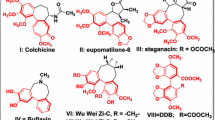
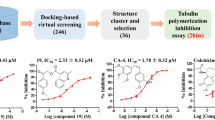
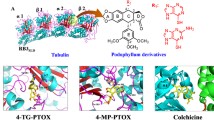
Our screen for tubulin-binding small molecules that do not depolymerize bulk cellular microtubules, but based upon structural features of well known microtubule-depolymerizing colchicine and podophyllotoxin, revealed tubulin binding anti-cancer property of noscapine (Ye et al. in Proc Natl Acad Sci USA 95:2280–2286, 1998). Guided by molecular modelling calculations and structure–activity relationships we conjugated at C9 of noscapine, a folate group—a ligand for cellular folate receptor alpha (FRα). FRα is over-expressed on some solid tumours such as ovarian epithelial cancers. Molecular docking experiments predicted that a folate conjugated noscapine (Targetin) accommodated well inside the binding cavity (docking score −11.295 kcal/mol) at the interface between α- and β-tubulin. The bulky folate moiety of Targetin is extended toward lumen of microtubules. The binding free energy (ΔG bind) computed based on molecular mechanics energy minimization was −221.01 kcal/mol that revealed favourable interaction of Targetin with the receptor. Chemical synthesis, tubulin-binding experiments, and anti-cancer activity in vitro corroborate fully well with the molecular modelling experiments. Targetin binds tubulin with a dissociation constant (K d value) of 149 ± 3.0 μM and decreases the transition frequencies between growth and shortening phases of microtubule assembly dynamics at concentrations that do not alter the total polymer mass. Cancer cells in general were more sensitive to Targetin compared with the founding compound noscapine (IC50 in the range of 15–40 μM). Quite strikingly, ovarian cancer cells (SKOV3 and A2780), known to overexpress FRα, were much more sensitive to targetin (IC50 in the range of 0.3–1.5 μM).













Similar content being viewed by others
References
Jordan MA, Wilson L (1999) The use and action of drugs in analyzing mitosis. Methods Cell Biol 61:267–295
Fuchs DA, Johnson RK (1978) Cytologic evidence that taxol, an antineoplastic agent from Taxus brevifolia, acts as a mitotic spindle poison. Cancer Treat Rep 62:1219–1222
Jordan MA, Toso RJ, Thrower D, Wilson L (1993) Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci USA 90:9552–9556
Reider C, Schultz A, Cole R, Sluder G (1994) Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol 127:1302–1310
Mathe G, Misset JL, De Vassal F, Gouveia J, Hayat M, Machover D, Belpomme D, Pico JL, Schwarzenberg L, Ribaud P, Musset M, Jasmin C, De Luca L (1978) Phase II clinical trial with vindesine for remission induction in acute leukemia, blastic crisis of chronic myeloid leukemia, lymphosarcoma, and hodgkin’s disease: absence of cross-resistance with vincristine. Cancer Treat Rep 62:805–809
Antoine EC, Rixe O, Auclerc G, Weil M, Khayat D (1997) Docetaxel: a review of its role in breast cancer treatment. Am J Clin Oncol 20:429–432
Chang AY, Kim K, Glick J, Anderson T, Karp D, Johnson D (1993) Phase II study of taxol, merbarone, and piroxantrone in stage IV non-small-cell lung cancer. J Natl Cancer Inst 85:346–347
McGovren JP (1994) Pharmacologic considerations in cancer chemotherapy. Cancer chemotherapy handbook. Appleton & Lange, Norwalk, pp 15–34
Calvaletti G, Tredici G, Braga M, Tazzari S (1995) Experimental peripheral neuropathy induced in adult rats by repeated intraperitoneal administration of taxol. Exper Neurol 133:64–72
Ye K, Ke Y, Keshava N, Shanks J, Kapp IA, Tekmal RR, Petros J, Joshi HC (1998) Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proc Natl Acad Sci USA 95:2280–2286
Landen JW, Lang R, McMahon SJ, Rusan NM, Yvon AM, Adams AW, Sorcinelli MD, Campbell R, Bonaccorsi P, Ansel JC, Archer DR, Wadsworth P, Armstrong CA, Joshi HC (2002) Noscapine alters microtubule dynamics in living cells and inhibits the progression of melanoma. Cancer Res 62:4109–4114
Zhou J, Panda D, Landen JW, Wilson L, Joshi HC (2002) Minor alteration of microtubule dynamics causes loss of tension across kinetochore pairs and activates the spindle checkpoint. J Biol Chem 277:17200–17208
Zhou J, Gupta K, Yao J, Ye K, Panda D, Giannakakou P, Joshi HC (2002) Paclitaxel-resistant human ovarian cancer cells undergo c-Jun NH2-terminal kinase-mediated apoptosis in response to noscapine. J Biol Chem 277:39777–39785
Dahlstrom B, Mellstrand T, Lofdahl CG, Johansson M (1982) Pharmakokinetic properties of noscapine. Eur J Clin Pharmacol 22:535–539
Karlsson MO, Dahlstrom B, Eckernas SA, Johansson M, Alm AT (1990) Pharmacokinetics of oral noscapine. Eur J Clin Pharmacol 39:275–279
Aneja R, Dhiman N, Idnani J, Awasthi A, Arora SK, Chandra R, Joshi HC (2007) Preclinical pharmacokinetics and bioavailability of noscapine, a tubulin-binding anticancer agent. Cancer Chemother Pharmacol 60(6):831–839
Ke Y, Ye K, Grossniklaus HE, Archer DR, Joshi HC, Kapp JA (2000) Noscapine inhibits tumor growth with little toxicity to normal tissues or inhibition of immune responses. Cancer Immunol Immunother 49:217–225
Aneja R, Vangapandu SN, Lopus M, Chandra R, Panda D, Joshi HC (2006) Development of a novel nitro-derivative of noscapine for the potential treatment of drug-resistant ovarian cancer and T-cell lymphoma. Mol Pharmacol 69:1801–1809
Aneja R, Vangapandu SN, Lopus M, Viswesarappa VG, Dhiman N, Verma A, Chandra R, Panda D, Joshi HC (2006) Synthesis of microtubule-interfering halogenated noscapine analogs that perturb mitosis in cancer cells followed by cell death. Biochem Pharmacol 72:415–426
Naik PK, Chatterji BP, Vangapandu SN, Aneja R, Chandra R, Kanteveri S, Joshi HC (2011) Rational design, synthesis and biological evaluations of amino-noscapine: a high affinity tubulin-binding noscapinoid. J Comput Aided Drug Des 25(5):443–454
Naik PK, Santoshi S, Rai A, Joshi HC (2011) Molecular modelling and competition binding study of Br- noscapine and colchicine provide insight into noscapinoid-tubulin binding site. J Mol Graph Model 29:947–955
Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP (2005) Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem 338:284–293
Ross JF, Chaudhuri PK, Ratnam M (1994) Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer 73:2432–2443
Ravelli RB, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M (2004) Insight into tubulin regulation from a complex with colchicines and a stathmin-like domain. Nature 428:198–202
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47:1739–1749
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 47:1750–1759
Eldridge MD, Murray CW, Auton TR, Paolini GV, Mee RP (1997) Empirical scoring functions: I. The development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J Comput Aided Mol Des 11:425–445
Bag S, Tawari NR, Degani MD, Queener SF (2010) Design, synthesis, biological evaluation and computational investigation of novel inhibitors of dihydrofolate reductase of opportunistic pathogens. Bioorg Med Chem 18:3187–3197
Alam MA, Naik PK (2009) Molecular modelling evaluations of the cytotoxic activity of podophyllotoxin analogues. J Comput Aided Mol Des 23:209–225
Zhou R, Frienser RA, Ghosh A, Rizzo RC, Jorgensen WL, Levy RM (2001) New linear interaction method for binding affinity calculations using a continuum solvent model. J Phys Chem B 105:10388–10397
Alam MA, Naik PK (2009) Applying linear interaction energy method for binding affinity calculations of podophyllotoxin analogues with tubulin using continuum solvent model and prediction of cytotoxic activity. J Mol Graph Model 27:930–943
Guo W, Lee T, Sudimack J, Lee R (2000) Receptor-specific delivery of liposomes via folate-PEG-chol. J Liposome Res 10(2&3):179–195
Hamel E, Lin CM (1981) Glutamate-induced polymerization of tubulin: characteristics of the reaction and application to the large-scale purification of tubulin. Arch Biochem Biophys 209:29–40
Panda D, Chakrabarti G, Hudson J, Pigg K, Miller HP, Wilson L, Himes RH (2000) Suppression of microtubule dynamic instability and treadmilling by deuterium oxide. Biochemistry 39:5075–5081
Zhou J, Gupta K, Aggarwal S, Aneja R, Chandra R, Panda D, Joshi HC (2003) Brominated derivatives of noscapine are potent microtubule-interfering agents that perturb mitosis and inhibit cell proliferation. Mol Pharmacol 63:799–807
Andreu JM, Timasheff SN (1982) Tubulin bound to colchicine forms polymers different from microtubules. Proc Natl Acad Sci USA 79:6753–6756
Aneja R, Asress S, Dhiman N, Awasthi A, Rida PCG, Arora SK, Zhou J, Glass JD, Joshi HC (2010) Non-toxic melanoma therapy by a novel tubulin-binding agent. Int J Cancer 126:256–265
Belmont LD, Hyman AA, Sawin KE, Mitchison TJ (1990) Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell 62:579–589
Gliksman NR, Skibbens RV, Salmon ED (1993) How the transition frequencies of microtubule dynamic instability (nucleation, catastrophe, and rescue) regulate microtubule dynamics in interphase and mitosis: analysis using a Monte Carlo computer simulation. Mol Biol Cell 4:1035–1050
Kalli KR, Oberg AL, Keeney GL, Christianson TJ, Low PS, Knutson KL, Hartmann LC (2008) Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol 108:619–626
Acknowledgments
We thank Dr. Dulal panda, IIT Bombay, India, for his support and sharing technical details of Targetin-tubulin interactions and microtubule polymerization assays. Grant supports: NIH grants CA-095317-01A2 (H.C. Joshi) and BOYSCAST fellowship (SR/BY/L-37/09; Department of Science and Technology, Government of India) to Pradeep K. Naik.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Naik, P.K., Lopus, M., Aneja, R. et al. In silico inspired design and synthesis of a novel tubulin-binding anti-cancer drug: folate conjugated noscapine (Targetin). J Comput Aided Mol Des 26, 233–247 (2012). https://doi.org/10.1007/s10822-011-9508-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-011-9508-z