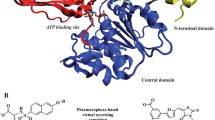
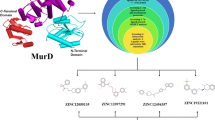
Abstract
The biosynthetic pathway of the bacterial peptidoglycan, where MurD is an enzyme involved at the intracellular stage of its construction, represents a collection of highly selective macromolecular targets for novel antibacterial drug design. In this study as part of our investigation of the MurD bacterial target two recently discovered classes of the MurD ligase inhibitors were investigated resulting from the lead optimization phases of the N-sulfonamide d-Glu MurD inhibitors. Molecular dynamics simulations, based on novel structural data, in conjunction with the linear interaction energy (LIE) method suggested the transferability of our previously obtained LIE coefficients to further d-Glu based classes of MurD inhibitors. Analysis of the observed dynamical behavior of these compounds in the MurD active site was supported by static drug design techniques. These results complement the current knowledge of the MurD inhibitory mechanism and provide valuable support for the d-Glu paradigm of the inhibitor design.









Similar content being viewed by others
References
Rice LB (2006) Unmet medical needs in antibacterial therapy. Biochem Pharmacol 71:991–995
Brown ED, Wright GD (2005) New targets and screening approaches in antimicrobial drug discovery. Chem Rev 105:759–774
Vollmer W, Blanot D, de Pedro MA (2008) Peptidoglycan structure and architecture. FEMS Microbiol Rev 32:149–167
Smith CS (2006) Structure, function and dynamics in the mur family of bacterial cell wall ligases. J Mol Biol 362:640–655
Bertrand JA, Auger G, Martin L, Fanchon E, Blanot D, Le Beller D, van Heijenoort J, Dideberg O (1999) Determination of the MurD mechanism through crystallographic analysis of enzyme complexes. J Mol Biol 289:579–590
Anderson MS, Eveland SS, Onishi H, Pompliano DL (1996) Kinetic mechanism of the Escherichia coli UDPMurNAc-tripeptide d-alanyl-d-alanine-adding enzyme: use of a glutathione S-transferase fusion. Biochemistry 35:16264–16269
Emanuele JJ Jr, Jin H, Yanchunas J Jr, Villafranca JJ (1997) Evaluation of the kinetic mechanism of Escherichia coli uridine diphosphate-N-acetylmuramate:l-alanine ligase. Biochemistry 36:7264–7271
Perdih A, Hodoscek M, Solmajer T (2009) MurD ligase from E. coli: Tetrahedral intermediate formation study by hybrid quantum mechanical/molecular mechanical replica path method. Proteins: Struct Funct Bioinf 74:744–759
Bertrand JA, Fanchon E, Martin L, Chantalat L, Auger G, Blanot D, van Heijenoort J, Dideberg O (2000) “Open” structures of MurD: domain movements and structural similarities with folylpolyglutamate synthetase. J Mol Biol 301:1257–1266
Perdih A, Kotnik M, Hodoscek M, Solmajer T (2007) Targeted molecular dynamics simulation studies of binding and conformational changes in E. Coli MurD. Proteins: Struct Funct Bioinf 68:243–254
Perdih A, Solmajer T (2012) MurD ligase from E. coli: C-terminal domain closing motion. Comput Theor Chem 979:73–81
Zoeiby AE, Sanschagrin F, Levesque RC (2002) Structure and function of the Mur enzymes: development of novel inhibitors. Mol Microbiol 47:1–12
Tanner ME, Vaganay S, van Heijenoort J, Blanot D (1996) Phosphinate inhibitors of the d-glutamic acid-adding enzyme of peptidoglycan biosynthesis. J Org Chem 61:1756–1760
Kotnik M, Humljan J, Contreras-Martel C, Oblak M, Kristan K, Hervé M, Blanot D, Urleb U, Gobec S, Dessen A, Solmajer T (2007) Structural and functional characterization of enantiomeric glutamic acid derivatives as potential transition state analogue inhibitors of MurD ligase. J Mol Biol 370:107–115
Perdih A, Bren U, Solmajer T (2009) Binding free-energy calculations of N-sulphonyl glutamic acid inhibitors of MurD ligase. J Mol Model 15:983–996
Zidar N, Tomasic T, Sink R, Rupnik V, Kovac A, Turk S, Patin D, Blanot D, Contreras Martel C, Dessen A, Müller Premru M, Zega A, Gobec S, Peterlin Masic L, Kikelj D (2010) Discovery of novel 5-benzylidenerhodanine and 5-benzylidenethiazolidine-2,4-dione inhibitors of MurD ligase. J Med Chem 53:6584–6594
Tomašić T, Zidar N, Šink R, Kovač A, Blanot D, Contreras-Martel C, Dessen A, Müller-Premru M, Zega A, Gobec S, Kikelj D, Peterlin Mašič L (2011) Structure-based design of a new series of d-glutamic acid based inhibitors of bacterial UDP-N-acetylmuramoyl-l-alanine:d-glutamate ligase (MurD). J Med Chem 54:4600–4610
Sosič I, Barreteau H, Simčič M, Sink R, Cesar J, Zega A, Grdadolnik SG, Contreras-Martel C, Dessen A, Amoroso A, Joris B, Blanot D, Gobec S (2011) Second-generation sulphonamide inhibitors of d-glutamic acid-adding enzyme: activity optimisation with conformationally rigid analogues of d-glutamic acid. Eur J Med Chem 46:2880–2894
Perdih A, Kovač A, Wolber G, Blanot D, Gobec S, Solmajer T (2009) Discovery of novel benzene 1,3-dicarboxylic acid inhibitors of bacterial MurD and MurE ligases by structure-based virtual screening approach. Bioorg Med Chem Lett 19:2668–2673
Åqvist J, Medina C, Samuelson JE (1994) A new method for predicting binding affinity in computer-aided drug design. Protein Eng 7:385–391
Åqvist J, Luzhkov VB, Brandsdal BO (2002) Ligand binding affinities from MD simulations. Acc Chem Res 35:358–365
Marelius J, Kolmodin K, Feierberg I, Åqvist J (1998) A molecular dynamics program for free energy calculations and empirical valence bond simulations in biomolecular systems. J Mol Graph Model 16:213–225
Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz M Jr, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA (1995) A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc 117:5179–5197
Gaussian 09, Revision A.1, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ, Gaussian, Inc., Wallingford CT, 2009
Bayly CI, Cieplak P, Cornell WD, Kollman PA (1993) A well-behaved electrostatic potential based method using charge restrains for deriving atomic charges: the RESP model. J Phys Chem 97:10269–10280
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
King G, Warshel A (1989) A surface constrained all-atom solvent model for effective simulations of polar solutions. J Chem Phys 91:3647–3661
Lee FS, Warshel A (1992) A local reaction field method for fast evaluation of long-range electrostatic interactions in molecular simulations. J Chem Phys 97:3100–3107
Handler N, Brunhofer G, Studenik C, Leisser K, Jaeger W, Parth S, Erker T (2007) ‘Bridged’ stilbene derivatives as selective cyclooxygenase-1 inhibitors. Bioorg Med Chem 15:6109–6118
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Christ CD, Mark AE, van Gunsteren WF (2010) Basic ingredients of free energy calculations: a review. J Comput Chem 31:1569–1582
Chen X, Tropsha A (2006) Calculation of the relative binding affinity of enzyme inhibitors using the generalized linear response method. J Chem Theory Comput 2:1435–1443
Carlson J, Boukharta L, Aquist J (2008) Combining docking, molecular dynamics and the linear interaction energy method to predict binding modes and affinities for non-nucleoside inhibitors to HIV-1 reverse transcriptase. J Med Chem 51:2648–2656
Kolb P, Huang D, Dey F, Caflisch A (2008) Discovery of kinase inhibitors by high-throughput docking and scoring based on a transferable linear interaction energy model. J Med Chem 51:1179–1188
Bortolato A, Moro S (2007) In silico binding free energy predictability by using the linear interaction energy (LIE) method: bromobenzimidazole CK2 inhibitors as a case study. J Chem Inf Model 47:572–582
Bren M, Florián J, Mavri J, Bren U (2007) Do all pieces make a whole? Thiele cumulants and the free energy decomposition. Theor Chem Acc 117:535–540
Baell JB, Holloway GA (2010) New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem 53:2719–2740
Mendgen T, Steuer C, Klein CD (2011) Privileged scaffolds or promiscuous binders—a comparative study on rhodanines and related heterocycles in medicinal chemistry. J Med Chem 55:743–753
McGovern SL, Stoichet BK (2003) Kinase inhibitors: not just for kinases anymore. J Med Chem 46:1478–1783
Baum B, Muley L, Smolinski M, Heine A, Hangauer D, Klebe G (2010) Non-additivity of functional group contributions in protein-ligand binding: a comprehensive study by crystallography and isothermal titration calorimetry. J Mol Biol 397:1042–1054
Patel Y, Gillet VJ, Howe T, Pastor J, Oyarzabal J, Willett P (2008) Assessment of additive/nonadditive effects in structure-activity relationships: implications for iterative drug design. J Med Chem 51:7552–7562
Kuhn B, Fuchs JE, Reutlinger M, Stahl M, Taylor NR (2011) Rationalizing tight ligand binding through cooperative interaction networks. J Chem Inf Model 51:3180–3198
Goodford PJ (1985) A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. J Med Chem 28:857–864
Kollman PA (1993) Free energy calculations: applications to chemical and biochemical phenomena. Chem Rev 93:2395–2417
Wolber G, Langer T (2005) LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J Chem Inf Model 45:160–169
Wolber G, Dornhofer A, Langer T (2006) Efficient overlay of small organic molecules using 3D pharmacophores. J Comput Aided Mol Design 20:773–788
Acknowledgments
This work was supported by the postdoctoral grant of the Ministry of Higher Education, Science and Technology of the Republic of Slovenia (Grant Number: Z1-4111).The authors would like thank Dr. Jernej Zidar and Barbara Pogorelčnik from the National institute of Chemistry, Slovenia, for their helpful technical assistance and Dr. Yasmin Aristei from Molecular Discovery Ltd. for GRID map calculations. Dr. Urban Bren from the National Institute of Chemistry, Ljubljana is acknowledged for introducing us to LIE methodology in our previous binding study of MurD inhibitors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (MPG 10252 kb)
Supplementary material 3 (MPG 9886 kb)
Rights and permissions
About this article
Cite this article
Perdih, A., Wolber, G. & Solmajer, T. Molecular dynamics simulation and linear interaction energy study of d-Glu-based inhibitors of the MurD ligase. J Comput Aided Mol Des 27, 723–738 (2013). https://doi.org/10.1007/s10822-013-9673-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-013-9673-3