Abstract
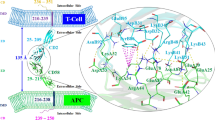
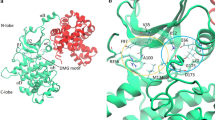
The CD2–CD58 protein–protein interaction is known to favor the recognition of antigen presenting cells by T cells. The structural, energetics, and dynamical properties of three known cyclic CD58 ligands, named P6, P7, and RTD-c, are studied through molecular dynamics (MD) simulations and molecular docking calculations. The ligands are built so as to mimic the C and F β-strands of protein CD2, connected via turn inducers. The MD analyses focus on the location of the ligands with respect to the experimental binding site and on the direct and water-mediated hydrogen bonds (H bonds) they form with CD58. Ligand P6, with a sequence close to the experimental β-strands of CD2, presents characteristics that explain its higher experimental affinity, e.g., the lower mobility and flexibility at the CD58 surface, and the larger number and occurrence frequency of ligand-CD58 H bonds. For the two other ligands, the structural modifications lead to changes in the binding pattern with CD58 and its dynamics. In parallel, a large set of molecular docking calculations, carried out with various search spaces and docking algorithms, are compared to provide a consensus view of the preferred ligand binding modes. The analysis of the ligand side chain locations yields results that are consistent with the CD2–CD58 crystal structure and suggests various binding modes of the experimentally identified hot spot of the ligands, i.e., Tyr86. P6 is shown to form a number of contacts that are also present in the experimental CD2–CD58 structure.











Similar content being viewed by others
References
Sable R, Jois J (2015) Surfing the protein-protein interaction surface using docking methods: application to the design of PPI inhibitors. Molecules 20:11569–11603
Zinzalla G, Thurston DE (2009) Targeting protein–protein interactions for therapeutic intervention: a challenge for the future. Future Med Chem 1:65–93
Wang JH, Smolyar A, Tan K, Liu JH, Kim M, Sun ZY, Wagner G, Reinherz EL (1999) Structure of a heterophilic adhesion complex between the human CD2 and CD58 (LFA-3) counter receptors. Cell 97:791–803
Raychaudhuri S, Thomson BP, Remmers EF, Eyre S, Hinks A, Guiducci C, Catanese JJ, Xie G, Stahl EA, Chen R, Alfredsson L, Amos CI, Ardlie KG, Consortium BIRAC, Barton A, Bowes J, Burtt NP, Chang M, Coblyn J, Costenbader KH, Criswell LA, Crusius JB, Cui J, De Jager PL, Ding B, Emery P, Flynn E, Harrison P, Hocking LJ, Huizinga TW, Kastner DL, Ke X, Kurreeman FA, Lee AT, Liu X, Li Y, Martin P, Morgan AW, Padyukov L, Reid DM, Seielstad M, Seldin MF, Shadick NA, Steer S, Tak PP, Thomson W, van der Helm-van Mil AH, van der Horst-Bruinsma IE, Weinblatt ME, Wilson AG, Wolbink GJ, Wordsworth P, YEAR Consortium, Altshuler D, Karlson EW, Toes RE, de Vries N, Begovich AB, Siminovitch KA, Worthington J, Klareskog L, Gregersen PK, Daly MJ, Plenge RM (2009) Genetic variants at CD28, PRDM1 and CD2/CD58 are associated with rheumatoid arthritis risk. Nat Genet 41:1313–1320
Liu J, Li C, Ke S, Satyanarayanajois SD (2007) Structure-based rational design of β-hairpin peptides from discontinuous epitopes of cluster of differentiation 2 (CD2) protein to modulate cell adhesion interaction. J Med Chem 50:4038–4047
Gokhale A, Weldeghiorghis ThK, Taneja V, Satyanarayanajois D (2011) Conformationally constrained peptides from CD2 to modulate protein-protein interactions between CD2 and CD58. J Med Chem 54:5307–5319
Ikemizu S, Sparks LM, van der Merwe PA, Harlos K, Stuart DI, Jones EY, Davis SJ (1999) Crystal structure of the CD2-binding domain of CD58 (lymphocyte function-associated antigen 3) at 1.8-A resolution. Proc Natl Acad Sci USA 96:4289–4294
van der Merwe PA, Barclay AN, Mason DW, Davies EA, Morgan BP, Tone M, Krishnam AK, Ianelli C, Davis SJ (1994) Human cell-adhesion molecule CD2 binds CD58 (LFA-3) with a very low affinity and an extremely fast dissociation rate but does not bind CD48 or CD59. Biochemistry 33:10149–10160
Kim M, Sun ZYJ, Byron O, Campbell G, Wagner G, Wang JH, Reinherz EL (2001) Molecular dissection of the CD2–CD58 counter-receptor interface identifies CD2 Tyr86 and CD58 Lys34 residues as the functional “hot spot”. J Mol Biol 312:711–720
Gokhale A, Kanthala S, Latendresse J, Taneja V, Satyanarayana SD (2013) Immunosuppression by co-stimulatory molecules: inhibition of CD2–CD48/CD58 interaction by peptides from CD2 to suppress progression of collagen-induced arthritis in mice. Chem Biol Drug Des 82:106–118
Gokhale AS, Sable R, Walker JD, McLaughlin L, Kousoulas KG, Satyanarayana SD (2015) Inhibition of cell adhesion and immune responses in the mouse model of collagen-induced arthritis with a peptidomimetic that blocks CD2–CD58 interface interactions. Biopolymers 104:733–742
Sable R, Durek T, Taneja V, Craik DJ, Pallerla S, Gauthier T, Jois S (2016) Constrained cyclic peptides as immunomodulatory inhibitors of the CD2:CD58 protein-protein interaction. ACS Chem Biol 11:2366–2374
Bayas MV, Schulten K, Leckband D (2003) Forced detachment of the CD2–CD58 complex. Biophys J 84:2223–2233
Bayas MV, Kearney A, Avramovic A, van der Merwe PA, Leckband DE (2007) Impact of salt bridges on the equilibrium binding and adhesion of human CD2 and CD58. J Biol Chem 282:5589–5596
Abdel-Azeim S, Chermak E, Vangone A, Oliva R, Cavallo L (2014) MDcons: Intermolecular contact maps as a tool to analyze the interface of protein complexes from molecular dynamics trajectories. Bioinformatics 15:S1
Wang X, Ji CG, Zhang ZH (2015) Glycosylation modulates human CD2–CD58 adhesion via conformational adjustment. J Phys Chem B 119:6493–6501
Jining L, Makagiansar I, Yusuf-Makagiansar H, Chow VTK, Siahaan TJ, Jois SDS (2004) Design, structure and biological activity of β-turn peptides of CD2 protein for inhibition of T-cell adhesion. Eur J Biochem 271:2873–2886
Lawson ADG, MacCoss M, Heer JP (2018) Importance of rigidity in designing small molecules drugs to tackle protein-protein interactions (PPIs) through stabilization of desired conformers. J Med Chem 61:4283–4289
PDBePISA (2018) Proteins, Interfaces, structures and assemblies v.1.52. http://www.ebi.ac.uk/msd-srv/prot_int/cgi-bin/piserver. Accessed 26 Feb 2018
Bernstein FC, Koetzle TF, Williams GJB, Meyer EF Jr, Brice MD, Rodgers JR, Kennard O, Shimanouchi T, Tasumi M (1977) The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol 112:535–542
Pietal MJ, Tuszynska I, Bujnicki JM (2007) PROTMAP2D: visualization, comparison and analysis of 2D maps of protein structure. Bioinformatics 23:1429–1430
PyMol™ M (2013) Graphics System, v.1.8.6.0. Schrödinger LLC, New York
Hess B, Kutzner C, van der Spoel D, Lindahl E (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4:435–447
Pronk S, Páll S, Schulz R, Larsson P, Bjelkmar P, Apostolov R, Shirts MR, Smith JC, Kasson PM, van der Spoel D, Hess B, Lindahl E (2013) GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29:845–854
Showalter SA, Brüschweiler R (2007) Validation of molecular dynamics simulations of biomolecules using NMR spin relaxation as benchmarks: application to the AMBER99SB force field. J Chem Theory Comput 3:961–975
Darré L, Tek A, Baaden M, Pantano S (2012) Mixing atomistic and coarse grain solvation models for MD simulations: let WT4 handle the bulk. J Chem Theory Comput 8:3880–3894
Gonzales HC, Darré L, Pantano S (2013) Transferable mixing of atomistic and coarse-grained water models. J Phys Chem B 117:14438–14448
Darré L, Machado MR, Brandner AF, González HC, Ferreira S, Pantano S (2015) SIRAH: a structurally unbiased coarse-grained force field for proteins with aqueous solvation and long-range electrostatics. J Chem Theory Comput 11:723–739
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) Autodock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 16:2785–2791
Koes DR, Baumgartner MP, Camacho CJ (2013) Lessons learned in empirical scoring with smina from the CSAR 2011 benchmarking exercise. J Chem Inf Model 53:1893–1904
Trott O, Olson AJ (2010) AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 31:455–461
Jones G, Willett P, Glen RC, Leach AR, Taylor R (1997) Development and validation of a genetic algorithm for flexible docking. J Mol Biol 267:727–748
Pierce BG, Hourai Y, Weng Z (2011) Accelerating protein docking in ZDOCK using an advanced 3D convolution library. PLoS One 6:e24657
Ramírez-Aportela E, López-Blanco JR, Chacón P (2016) FRODOCK 2.0: fast protein-protein docking server. Bioinformatics 32:2386–2388
Kozakov D, Hall DR, Xia B, Porter KA, Padhorny D, Yueh C, Beglov D, Vajdab S (2017) The ClusPro web server for protein-protein docking. Nat Protoc 12:255–278
van Zundert GCP, Rodrigues JPGLM, Trellet M, Schmitz C, Kastritis PL, Karaca E, Melquiond ASJ, van Dijk M, de Vries SJ, Bonvin AMJJ (2016) The HADDOCK2.2 webserver: user-friendly integrative modeling of biomolecular complexes. J Mol Biol 428:720–725
Forli S, Huey R, Pique ME, Sanner MF, Goodsell DS, Olson AJ (2016) Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat Protoc 11:905–919
Feinstein WP, Brylinski M (2015) Calculating an optimal box size for ligand docking and virtual screening against experimental and predicted binding pockets. J Chem Inform 7:18
Bizzarri AR, Cannistraro S (2002) Molecular dynamics of water at the protein-solvent interface. J Phys Chem B 106:6617–6633
Dastidar SG, Mukhopadhyay C (2003) Structure, dynamics, and energetics of water at the surface of a small globular protein: a molecular dynamics simulation. Phys Rev E 68:021921
Leherte L, Vercauteren DP (2014) Evaluation of reduced point charge models of proteins through molecular dynamics simulations: application to the Vps27 UIM-1—ubiquitin complex. J Mol Graphics Model 47:44–61
Acknowledgements
The authors thank the reviewers for their comments that helped to improve the manuscript, as well as Dr. Jose Ceron-Carrasco and Horacio Pérez Sánchez for numerous discussions. Frédéric Wautelet and Laurent Demelenne are gratefully acknowledged for program installation and maintenance. The research used resources of the ‘Plateforme Technologique de Calcul Intensif (PTCI)’ (http://www.ptci.unamur.be) located at the University of Namur, Belgium, which is supported by the F.R.S.-FNRS convention 2.5020.11. The PTCI is member of the ‘Consortium des Équipements de Calcul Intensif (CÉCI)’ (http://www.ceci-hpc.be). This research used as well French resources of (1) the GENCI-CINES/IDRIS (Grants A0020805117) and (2) CCIPL (Centre de Calcul Intensif des Pays de Loire). ADL thanks the ‘Région Pays de la Loire (Dynamique scientifique Piramid)’ for the support. The authors also thank the Interuniversity Attraction Pole program no. 7/05: ‘Functional supramolecular systems’ initiated by the Belgian Science Policy Office. Funding was provided by the Wallonie-Bruxelles International WBI (PHC Tournesol DoIFAD) and the Belgian National Foundation for Scientific Research (FNRS), by the French Ministry of Foreign and European Affairs, and by the Ministry of Higher Education and Research, in the framework of the Hubert Curien partnerships (PHC Tournesol #40638PL).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leherte, L., Petit, A., Jacquemin, D. et al. Investigating cyclic peptides inhibiting CD2–CD58 interactions through molecular dynamics and molecular docking methods. J Comput Aided Mol Des 32, 1295–1313 (2018). https://doi.org/10.1007/s10822-018-0172-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-018-0172-4