Abstract
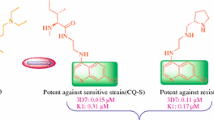
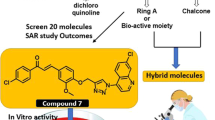
The quinolinyl chalcones series (A1–A14) were screened for antimalarial activity. According to in vitro antimalarial studies, many quinolinyl chalcones are potentially active against CQ-sensitive and resistance P. falciparum strains with no toxicity against Vero cell lines. The most active quinolinyl chalcones A4 (with IC50 0.031 μM) made a stable A4–heme complex with − 25 kcal/mole binding energy and also showed strong π–π interaction at 3.5 Å. Thus, the stable A4–heme complex formation suggested that these quinolinyl chalcones act as a blocker for heme polymerization. The docking results of quinolinyl chalcones with Pf-DHFR showed that the halogenated benzene part of quinolinyl chalcones made strong interaction with Pf-DHFR as compared to quinoline part. A strong A4–Pf-DHFR complex was formed with low binding energy (− 11.04 kcal/mole). The ADMET properties of quinolinyl chalcones were also studied. The in vivo antimalarial studies also confirmed the A4 as an active antimalarial agent.
Graphical abstract










Similar content being viewed by others
References
Breman JG, Egan A, Keusch GT (2001) Am J Trop Med Hyg 64:1–11
Mendis K, Sina B, Marchesini P, Carter R (2001) Am J Trop Med Hyg 64:97–106
Gessler MC, Nkunya MHH, Mwasumbi LB, Heinrich M, Tanner M (1994) Acta Trop 56(1):65–77
Tran QL, Tezuka Y, Ueda J-Y, Nguyen NT, Maruyama Y, Begum K, Kim H-S, Wataya Y, Tran QK, Kadota S (2003) Ethnopharmacol 86(2):249–252
Domínguez JN, León C, Rodrigues J, de Gamboa Domínguez N, Gut J, Rosenthal PJ (2005) J Med Chem 48(10):3654–3658
Aponte JJ, Aide P, Renom M, Mandomando I, Bassat Q, Sacarlal J, Manaca MN, Lafuente S, Barbosa A, Leach A, Lievens M, Vekemans J, Sigauque B, Dubois M-C, Demoitié M-A, Sillman M, Savarese B, McNeil JG, Macete E, Ballou WR, Cohen J, Alonso PL (2007) Lancet 370(9598):1543–1551
Vekemans J, Marsh K, Greenwood B, Leach A, Kabore W, Soulanoudjingar S, Asante KP, Ansong D, Evans J, Sacarlal J, Bejon P, Kamthunzi P, Salim N, Njuguna P, Hamel MJ, Otieno W, Gesase S, Schellenberg D (2011) Malaria J 10(1):221
White NJ (2004) J Clin Invest 113(8):1084–1092
Domı́nguez JN, Charris JE, Lobo G, de Gamboa Domı́nguez N, Moreno MAM, Riggione F, Sanchez E, Olson J, Rosenthal PJ (2001) Euro J Med Chem 36(6):555–560
Bohle DS, Conklin BJ, Cox D, Madsen SK, Paulson S, Stephens PW, Yee GT (1994) Am Chem Soc Symp Ser 572:497–515
Slater A, Cerami A (1992) Nature 355(6356):167
Sullivan DJ, Gluzman IY, Russell DG, Goldberg DE (1996) Proc Natl Acad Sci USA 93(21):11865–11870
Tilley L, Loria P, Foley M (2001) Chloroquine and other quinoline antimalarials. Antimalarial chemother. Springer, Berlin, pp 87–121
Hameed A, Abdullah MI, Ahmed E, Sharif A, Irfan A, Masood S (2016) Bioorg Chem 65:175–182
Abdullah MI, Mahmood A, Madni M, Masood S, Kashif M (2014) Bioorg Chem 54:31–37
Meth-Cohn O, Narine B, Tarnowski B (1981) J Chem Soc Perkin Trans 1:1520–1530
Herencia F, Ferrándiz ML, Ubeda A, Domínguez J, Charris JE, Lobo GM, Alcaraz MJ (1998) Bioorg Med Chem Lett 8(10):1169–1174
Li R, Kenyon GL, Cohen FE, Chen X, Gong B, Dominguez JN, Davidson E, Kurzban G, Miller RE, Nuzum EO, Rosenthal PJ, McKerrow JH (1995) J Med Chem 38(26):5031–5037
Becke AD (1988) Phys Rev A 38(6):3098–3100
Lee C, Yang W, Parr RG (1988) Phys Rev B 37(2):785–789
Koenig D (1965) Acta Crystallogr 18(4):663–673
Schäfer A, Huber C, Ahlrichs R (1994) J Chem Phys 100(8):5829–5835
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ. Gaussian 16 Rev. B.01. Wallingford, CT; 2016
Bali A (2011) ISRN Pharm 2011:5
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Adv Drug Deliv Rev 23(1):3–25
Ertl P, Rohde B, Selzer P (2000) J Med Chem 43(20):3714–3717
Nikolova N, Jaworska J (2003) QSAR Comb Sci 22(9–10):1006–1026
Glen RC, Adams SE (2006) QSAR Comb Sci 25(12):1133–1142
Bender A, Glen RC (2004) Org Biomol Chem 2(22):3204–3218
Patterson DE, Cramer RD, Ferguson AM, Clark RD, Weinberger LE (1996) J Med Chem 39(16):3049–3059
Willett P, Barnard JM, Downs GM (1998) J Chem Inf Comput Sci 38(6):983–996
Xue CX, Cui SY, Liu MC, Hu ZD, Fan BT (2004) Eur J Med Chem 39(9):745–753
Gacche R, Khsirsagar M, Kamble S, Bandgar B, Dhole N, Shisode K, Chaudhari A (2008) Chem Pharm Bull 56(7):897–901
Kumar S, Guha M, Choubey V, Maity P, Bandyopadhyay U (2007) Life Sci 80(9):813–828
Weissbuch I, Leiserowitz L (2008) Chem Rev 108(11):4899–4914
Babu LT, Larry AW (2005) Comb Chem High Throughput Screen 8(1):63–79
Cohen SN, Phifer KO, Yielding KL (1964) Nature 202:805
Dorn A, Vippagunta SR, Matile H, Jaquet C, Vennerstrom JL, Ridley RG (1998) Biochem Pharmacol 55(6):727–736
Warhurst DC, Craig JC, Adagu IS, Guy RK, Madrid PB, Fivelman QL (2007) Biochem Pharmacol 73(12):1910–1926
Sommadossi JP, Carlisle R, Schinazi RF, Zhou Z (1988) Antimicrob Agents Chemother 32(7):997–1001
Schinazi RF, Peters J, Williams CC, Chance D, Nahmias AJ (1982) Antimicrob Agents Chemother 22(3):499–507
Chou J, Chou T-C (1985) Dose-effect analysis with microcomputers: quantitation of ED50, LD50, synergism, antagonism, low-dose risk, receptor binding and enzyme kinetics, A Computer Software for Apple II Series and IBMPC and Instruction Manual. Elsevier-Biosoft, Elsevier Science Publishers, Cambridge
Jain M, Khan SI, Tekwani BL, Jacob MR, Singh S, Singh PP, Jain R (2005) Bioorg Med Chem Lett 13(14):4458–4466
Makler M, Ries J, Williams J, Bancroft J, Piper R, Gibbins B, Hinrichs D (1993) Am J Trop Med Hyg 48(6):739–741
Makler MT, Hinrichs DJ (1993) Am J Trop Med Hyg 48(2):205–210
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hameed, A., Masood, S., Hameed, A. et al. Anti-malarial, cytotoxicity and molecular docking studies of quinolinyl chalcones as potential anti-malarial agent. J Comput Aided Mol Des 33, 677–688 (2019). https://doi.org/10.1007/s10822-019-00210-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-019-00210-2