Abstract
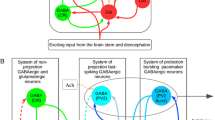
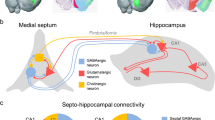
The medial septum-diagonal band (MSDB) complex is considered as a pacemaker for the hippocampal theta rhythm. Identification of the different cell types, their electro-physiological properties and their possible function in the generation of a synchronized activity in the MSDB is a hot topic. A recent electro-physiological study showed the presence of two antiphasically firing populations of parvalbumin containing GABAergic neurons in the MSDB. Other papers described a network of cluster-firing glutamatergic neurons, which is able to generate synchronized activity in the MSDB. We propose two different computer models for the generation of synchronized population theta oscillation in the MSDB and compare their properties. In the first model GABAergic neurons are intrinsically theta periodic cluster-firing cells; while in the second model GABAergic cells are fast-firing cells and receive periodic input from local glutamatergic neurons simulated as cluster-firing cells. Using computer simulations we show that the GABAergic neurons in both models are capable of generating antiphasic theta periodic population oscillation relying on local, septal mechanisms. In the first model antiphasic theta synchrony could emerge if GABAergic neurons form two populations preferentially innervate each other. In the second model in-phase synchronization of glutamatergic neurons does not require specific network structure, and the network of these cells are able to act as a theta pacemaker for the local fast-firing GABAergic circuit. Our simulations also suggest that neurons being non-cluster-firing in vitro might exhibit clustering properties when connected into a network in vivo.
Similar content being viewed by others
References
Bland BH, Oddie SD, Colom, LV (1999) Mechanisms of neural synchrony in the septohippocampal pathways underlying hippocampal theta generation. J. Neurosci. 19(8): 3223–3237.
Bor-hegyi Z, Varga V, Szilágyi N, Fabó D, Freund TF (2004) Phase segregation of medial septal GABA ergic neurons during hippocampal theta activity. J. Neurosci. 24(39): 8470–8479.
Bower JM, Beeman D (1998) The Book of GENESIS: Exploring Realistic Neural Models with the GEneral NEural SImulation System, 2 edition. Springer–Verlag.
Brashear HR, Zaborszky L, Heimer L (1986) Distribution of GABAergic and cholinergic neurons in the rat diagonal band. Neuroscience 17(2): 439–451.
Brazhnik ES, Fox SE (1997) Intracellular recordings from medial septal neurons during hippocampal theta rhythm. Exp. Brain. Res. 114(3): 442–453.
Brazhnik ES, Fox SE (1999) Action potentials and relations to the theta rhythm of medial septal neurons in vivo. Exp. Brain Res. 127(3): 244–258.
Cole AE, Nicoll RA (1984) Characterization of a slow cholinergic post-synaptic potential recorded in vitro from rat hippocampal pyramidal cells. J. Physiol. 352: 173–188.
Colom LV, Castaneda MT, Reyna T, Hernandez S, Garrido-Sanabria E (2005) Characterization of medial septal glutamatergic neurons and their projection to the hippocampus. Synapse 58(3): 151–164.
Destexhe A (2000) Modelling corticothalamic feedback and the gating of the thalamus by the cerebral cortex. J. Physiol. (Paris) 94: 391–410.
Dragoi G, Carpi D, Recce M, Csicsvári, J, Buzsáki G (1999) Interactions between hippocampus and medial septum during sharp waves and theta oscillation in the behaving rat. J. Neurosci. 19: 6191–99.
Dayan P. Abbott L (2001) Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems. MIT Press. Chapter 1: Neural encoding: Firing rates and spike statistics.
Ermentrout B (2002) Simulating, Analyzing, Animating Dynami cal Systems: A Guide to XPPAUT for Researchers and Students. SIAM, Philadelphia, PA.
Freund TF (1989) GABAergic septohippocampal neurons contain parvalbumin. Brain Res. 478(2): 375–381.
Freund TF, Antal M (1988) GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature 336(6195): 170–173.
Frotscher M, Leranth C (1985) Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: A combined light and electron microscopic study. J. Comp. Neurol. 239(2): 237–246.
Garner HL, Whittington MA, Henderson Z (2005) Induction by kainate of theta frequency rhythmic activity in the rat medial septum-diagonal band complex in vitro. J. Physiol. 564(Pt 1): 83–102.
Green JD, Arduini AA (1954) Hippocampal electrical activity in arousal. J. Neurophysiol 17: 533–557.
Griffith W (1988) Membrane properties of cell types within guinea pig basal forebrain nuclei in vitro. J. Neurophysiol 59(5): 1590–1612.
Griffith W Matthews R (1986) Electrophysiology of AChE-positive neurons in basal forebrain slices. Neurosci. Lett. 71(2): 169–174.
Griffith W, Sim J, Matthews R (1991) Electrophysiologic characteristics of basal forebrain neurons in vitro. Adv. Exp. Med. Biol. 295: 143–155.
Hajszan T, Alreja M, Leranth C (2004) Intrinsic vesicular glutamate transporter 2-immunoreactive input to septohippocampal parvalbumin-containing neurons: Novel glutamatergic local circuit cells. Hippocampus 14(4): 499–509.
Hasselmo ME, Hay J, Ilyn M, Gorchetchnikov A (2002) Neuromodulation, theta rhythm and rat spatial navigation. Neural Netw. 15(4–6): 689–707.
Henderson Z, Fiddler G, S Saha AB, Halasy K (2004) A parvalbumin-containing, axosomatic synaptic network in the rat medial septum: relevance to rhythmogenesis. Eur. J. Neurosci. 19(10): 2753–2798.
Henderson Z, Morris N, Grimwood P, Fiddler G, Yang H, Appenteng K (2001) Morphology of local axon collaterals of electrophysiologically characterised neurons in the rat medial septal/diagonal band complex. J. Comp. Neurol. 430(3): 410–432.
King C, Recce M, O'Keefe J (1998) The rhythmicity of cells of the medial septum/diagonal band of broca in the awake freely moving rat: Relationships with behaviour and hippocampal theta. European J. Neurosci. 10: 464–477.
Kiss J, Patel AJ, Baimbridge KG, Freund TF (1990) Topographical localization of neurons containing parvalbumin and choline acetyltransferase in the medial septum-diagonal band region of the rat. Neuroscience 36(1): 61–72.
Knapp J, Morris N, Henderson Z, Matthews R (2000) Electrophysiological characteristics of non-bursting, glutamate decarboxylase messenger RNA-positive neurons of the medial septum/diagonal band nuclei of guinea-pig and rat. Neuroscience 98(4): 661–668.
Lengyel M, Huhn Z, Erdi P (2005) Computational theories on the function of theta oscillations. Biol. Cybern. 92(6): 393–408.
Leung L, Shen B (2004) Glutamatergic synaptic transmission participates in generating the hippocampal EEG. Hippocampus. 14(4):510–525.
Manseau F, Danik M, Williams S (2005) A functional glutamatergic neurone network in the medial septum and diagonal band area. J. Physiol. 566(3): 865–884.
Morris NP, Harris SJ, Henderson Z (1999) Parvalbumin-immunoreactive, fast-spiking neurons in the medial septum/diagonal band complex of the rat: Intracellular recordings in vitro. Neuroscience 92(2): 589–600.
O'Keefe J. and Nadel L (1978) The Hippocampus as a Cognitive Map. Clarendon Press, Oxford.
Petsche H, Stumpf C, Gogolak G (1962) The significance of the rabbit's septum as a relay station between the mid-brain and the hippocampus. I The control of hippocampal arousal activity by the septum cells. Electroencephalogr. Clin. Neurophysiol. 14: 202–211.
Puma C, Bizot JC (1999) Hippocampal theta rhythm in anesthetized rats: Role of AMPA glutamate receptors. Neuroreport 10(11): 2297–2300.
Serafin M, Williams S, Khateb A, Fort P, Muhlethaler M (1996) Rhythmic firing of medial septum non-cholinergic neurons. Neuroscience 75(3): 671–675.
Sotty F, Danik M, Manseau F, Quirion R, Williams S (2003) Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: Novel implications for hippocampal rhythmicity. J. Physiol. 551: 927–943.
Stewart M, Fox, SE (1989) Two populations of rhythmically bursting neurons in rat medial septum are revealed by atropine. J. Neurophysiol. 61(5): 982–993.
Stewart M, Fox SE (1990) Do septal neurons pace the hippocampal theta rhythm?Trends Neurosci. 13(5): 163–168.
Stumpf C, Petsche H, Gogolak G (1962) The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. II. The differential influence of drugs upon both the septal cell firing pattern and the hippocampus theta activity. Electroencephalogr. Clin. Neurophysiol. 14: 212–219.
Vanderwolf CH (1969) Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr. Clin. Neurophysiol. 26(4): 407–418.
Vértes RP, Kocsis B (1997) Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience 81: 893–926.
Vinogradova O, Brazhnik E, Karanov A, Zhadina S (1980) Neuronal activity of the septum following various types of deafferentation. Brain Res. 187(2): 353–368.
Vinogradova OS (1995) Expression, control, probable functional significance of the neuronal theta-rhythm. Prog. Neurobiol. 45(6): 523–583.
Wainger B, DeGennaro M, Santoro B, Siegelbaum S, Tibbs G (2001) Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature 411(6839): 805–10.
Wang XJ (2002) Pacemaker neurons for the theta rhythm and their synchronization in the septohippocampal reciprocal loop. J. Neurophysiol. 87(2): 889–900.
Wang XJ, Buzsáki G (1996) Gamma oscillation by synaptic inhibitionin a hippocampal interneuronl network model. J. Neurosci. 16: 6402–6413.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: David Golomb
Rights and permissions
About this article
Cite this article
Ujfalussy, B., Kiss, T. How do glutamatergic and GABAergic cells contribute to synchronization in the medial septum?. J Comput Neurosci 21, 343–357 (2006). https://doi.org/10.1007/s10827-006-9082-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-006-9082-x