Abstract
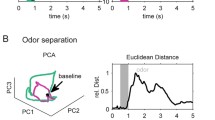
The antennal lobe (AL) is the primary structure within the locust’s brain that receives information from olfactory receptor neurons (ORNs) within the antennae. Different odors activate distinct subsets of ORNs, implying that neuronal signals at the level of the antennae encode odors combinatorially. Within the AL, however, different odors produce signals with long-lasting dynamic transients carried by overlapping neural ensembles, suggesting a more complex coding scheme. In this work we use a large-scale point neuron model of the locust AL to investigate this shift in stimulus encoding and potential consequences for odor discrimination. Consistent with experiment, our model produces stimulus-sensitive, dynamically evolving populations of active AL neurons. Our model relies critically on the persistence time-scale associated with ORN input to the AL, sparse connectivity among projection neurons, and a synaptic slow inhibitory mechanism. Collectively, these architectural features can generate network odor representations of considerably higher dimension than would be generated by a direct feed-forward representation of stimulus space.









Similar content being viewed by others
References
Ache, B., & Young, J. (2005). Olfaction: diverse species, conserved principles. Neuron, 48, 417–430. doi:10.1016/j.neuron.2005.10.022.
Axel, R. (1995). The molecular logic of smell. Scientific American, 273, 154–159.
Barbara, G., Zube, C., Rybak, J., Gauthier, M., & Grünewald, B. (2005). Acetylcholine, GABA and glutamate induce ionic currents in cultured antennal lobe neurons of the honeybee, Apis Mellifera. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology, 191, 823–836. doi:10.1007/s00359-005-0007-3.
Bazhenov, M., Timofeev, I., Steriade, M., & Sejnowski, T. (1998). Cellular and network models for intrathalamic augmenting responses during 10 Hz Stimulation. Journal of Neurophysiology, 79, 2730–2748.
Bazhenov, M., Stopfer, M., Rabinovich, M., Abarbanel, H., Sejnowski, T., & Laurent, G. (2001a). Model of cellular and network mechanisms for odor-evoked temporal patterning in the locust antennal lobe. Neuron, 30, 569–581. doi:10.1016/S0896-6273(01)00286-0.
Bazhenov, M., Stopfer, M., Rabinovich, M., Huerta, R., Abarbanel, H., Sejnowski, T., et al. (2001b). Model of transient oscillatory synchronization in the locust antennal lobe. Neuron, 30, 553–567. doi:10.1016/S0896-6273(01)00284-7.
Bhandawat, V., Olsen, S., Gouwens, N., Schlief, M., & Wilson, R. (2007). Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nature Neuroscience, 10, 1474–1482. doi:10.1038/nn1976.
Costanzo, R., & Morrison, E. (1989). Three-dimensional scanning electron microscopic study of the normal hamster olfactory epithelium. Journal of Neurocytology, 18, 381–391. doi:10.1007/BF01190841.
de Bruyne, M., Clyne, P., & Carlson, J. (1999). Odor coding in a model olfactory organ: the Drosophila Maxillary Palp. The Journal of Neuroscience, 19, 4520–4532.
de Bruyne, M., Foster, K., & Carlson, J. (2001). Odor coding in the drosophila antenna. Neuron, 30, 537–552. doi:10.1016/S0896-6273(01)00289-6.
Destexhe, A., Bal, T., McCormick, D., & Sejnowski, T. (1996). Ionic mechanisms underlying synchronized oscillations and propagating waves in a model of ferret thalamic slices. Journal of Neurophysiology, 76, 2049–2070.
Duchamp-Viret, P., Duchamp, A., & Chaput, M. (2000). Peripheral odor coding in the rat and frog: quality and intensity specification. The Journal of Neuroscience, 20, 2383–2390.
Dutar, P., & Nicoll, R. (1988). A physiological role for GABAB Receptors in the central nervous system. Nature, 332, 156–158. doi:10.1038/332156a0.
Friedrich, R., & Korsching, S. (1997). Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron, 18, 737–752. doi:10.1016/S0896-6273(00)80314-1.
Friedrich, R., & Laurent, G. (2001). Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science, 291, 889–894. doi:10.1126/science.291.5505.889.
Friedrich, R., & Laurent, G. (2004). Dynamics of olfactory bulb input and output activity during odor stimulation in zebrafish. Journal of Neurophysiology, 91, 2658–2669. doi:10.1152/jn.01143.2003.
Fuss, S., & Korsching, S. (2001). Odorant feature detection: activity mapping of stucture response relationships in the zebrafish olfactory bulb. The Journal of Neuroscience, 21, 8396–8407.
Galizia, C., Sachse, S., & Mustaparta, H. (2000). Calcium responses to pheromones and plant odours in the antennal lobe of the male and female moth Heliothis virescens. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology, 186, 1049–1063. doi:10.1007/s003590000156.
Gao, Q., Yuan, B., & Chess, A. (2000). Convergent projections of drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nature Neuroscience, 3, 780–785. doi:10.1038/75753.
Graziadei, P., & Metcalf, J. (1971). Autoradiographic and ultrastructural observations on the frog’s olfactory mucosa. Z Zellforsch Mikrosk Anat., 116, 305–318. doi:10.1007/BF00330630.
Hallem, E., & Carlson, J. (2006). Coding of odors by a receptor repertoire. Cell, 125, 143–160. doi:10.1016/j.cell.2006.01.050.
Hansson, B., & Anton, S. (2000). Function and morphology of the antennal lobe: new developments. Annual Review of Entomology, 45, 203–231. doi:10.1146/annurev.ento.45.1.203.
Heisenberg, M. (1998). What do the mushroom bodies do for the insect brain? An Introduction. Learning & Memory (Cold Spring Harbor, N.Y.), 5, 1–10.
Hildebrand, J., & Shepherd, G. (1997). Mechanisms of olfactory discrimination : converging evidence for common principles across phyla. Annual Review of Neuroscience, 20, 595–631. doi:10.1146/annurev.neuro.20.1.595.
Hildebrand, J., Rossler, W., & Tolbert, L. (1997). Postembryonic development of the olfactory system in the moth manduca sexta: primary-afferent control of glomerular development. Seminars in Cell & Developmental Biology, 8, 163–170. doi:10.1006/scdb.1996.0139.
Hodgkin, A., & Huxley, A. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. The Journal of Physiology, 117, 500–544.
Homberg, U., Christensen, T., & Hildebrand, J. (1989). Structure and function of the deutocerebrum in insects. Annual Review of Entomology, 34, 477–501. doi:10.1146/annurev.en.34.010189.002401.
Huguenard, J., Coulter, D., & McCormick, D. (1991). A fast transient potassium current in thalamic relay neurons. Kinetics of Activation and Inactivation. Journal of Neurophysiology, 66, 1305–1315.
Joerges, J., Küttner, A., Galizia, G., & Menzel, R. (1997). Representations of odours and odour mixtures visualized in the honeybee brain. Nature, 387, 285–288. doi:10.1038/387285a0.
Johnson, B., & Leon, M. (2000). Modular representation of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. The Journal of Comparative Neurology, 422, 496–509. doi:10.1002/1096-9861(20000710)422:4<496::AID-CNE2>3.0.CO;2-4.
Kenyon, F. (1896). The brain of the bee. A preliminary contribution to the morphology of the nervous system of the arthropoda. The Journal of Comparative Neurology, 6, 133–210. doi:10.1002/cne.910060302.
Laurent, G. (1996). Dynamical representation of odors by oscillating and evolving neural assemblies. Trends in Neurosciences, 19, 489–496. doi:10.1016/S0166-2236(96)10054-0.
Laurent, G., & Davidowitz, H. (1994). Encoding of olfactory information with oscillating neural assemblies. Science, 265, 1872–1875. doi:10.1126/science.265.5180.1872.
Laurent, G., & Naraghi, M. (1994). Odorant-induced oscillations in the mushroom bodies of the locust. The Journal of Neuroscience, 14, 2993–3004.
Laurent, G., Seymour-Laurent, K., & Johnson, K. (1993). Dendritic excitability and a voltage-gated calcium current in locust nonspiking local interneurons. Journal of Neurophysiology, 69, 1484–1498.
Laurent, G., Wehr, M., & Davidowitz, H. (1996). Temporal representations of odors in an olfactory network. The Journal of Neuroscience, 16, 3837–3847.
Laurent, G., Stopfer, M., Friedrich, R., Rabinovich, M., Volkovskii, A., & Abarbanel, H. (2001). Odor encoding as an active, dynamical process : experiments, computation, and theory. Annual Review of Neuroscience, 24, 263–297. doi:10.1146/annurev.neuro.24.1.263.
Leitch, B., & Laurent, G. (1996). GABAergic synapses in the antennal lobe and mushroom body of the locust olfactory system. The Journal of Comparative Neurology, 372, 487–514. doi:10.1002/(SICI)1096-9861(19960902)372:4<487::AID-CNE1>3.0.CO;2-0.
MacLeod, K., & Laurent, G. (1996). Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies. Science, 274, 976–979. doi:10.1126/science.274.5289.976.
MacLeod, K., Bäcker, A., & Laurent, G. (1998). Who reads temporal information contained across synchronized and oscillatory spike trains? Nature, 395, 693–698. doi:10.1038/27201.
Malnic, B., Hirono, J., Sato, T., & Buck, L. (1999). Combinatorial receptor codes for odors. Cell, 96, 713–723. doi:10.1016/S0092-8674(00)80581-4.
Mazor, O., & Laurent, G. (2005). Transient dynamics versus fixed points in odor representations by locust antennal lobe projection neurons. Neuron, 48, 661–673. doi:10.1016/j.neuron.2005.09.032.
Meister, M., & Bonhoeffer, T. (2001). Tuning and topography in an odor map on the rat olfactory bulb. The Journal of Neuroscience, 21, 1351–1360.
Mercer, A., & Hildebrand, J. (2002a). Developmental changes in the density of ionic currents in antennal-lobe neurons of the sphinx moth, Manduca Sexta. Journal of Neurophysiology, 87, 2664–2675.
Mercer, A., & Hildebrand, J. (2002b). Developmental changes in the electrophysiological properties and response characteristics of manduca antennal-lobe neurons. Journal of Neurophysiology, 87, 2650–2663.
Moulton, D. (1974). Dynamics of cell populations in the olfactory epithelium. Annals of the New York Academy of Sciences, 237, 52–61. doi:10.1111/j.1749-6632.1974.tb49843.x.
Ng, M., Roorda, R., Lima, S., Zemelman, B., Morcillo, P., & Miesenbock, G. (2002). Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron, 36, 463–474. doi:10.1016/S0896-6273(02)00975-3.
Perez-Orive, J., Mazor, O., Turner, G., Cassenaer, S., Wilson, R., & Laurent, G. (2002). Oscillations and sparsening of odor representations in the mushroom body. Science, 297, 359–365. doi:10.1126/science.1070502.
Perez-Orive, J., Bazhenov, M., & Laurent, G. (2004). Intrinsic and circuit properties favor coincidence detection for decoding oscillatory input. The Journal of Neuroscience, 24, 6037–6047. doi:10.1523/JNEUROSCI.1084-04.2004.
Reisenman, C., Christensen, T., Francke, W., & Hildebrand, J. (2004). Enantioselectivity of projection neurons innervating identified olfactory glomeruli. The Journal of Neuroscience, 24, 2602–2611. doi:10.1523/JNEUROSCI.5192-03.2004.
Rubin, B., & Katz, L. (1999). Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron, 23, 499–511. doi:10.1016/S0896-6273(00)80803-X.
Sachse, S., & Galizia, C. (2002). Role of inhibition for temporal and spatial odor representation in olfactory output neurons: a calcium imaging study. Journal of Neurophysiology, 87, 1106–1117.
Sachse, S., Rappert, A., & Galizia, C. (1999). The spatial representation of chemical structures in the antennal lobes of honeybees: steps towards the olfactory code. The European Journal of Neuroscience, 11, 3970–3982. doi:10.1046/j.1460-9568.1999.00826.x.
Sivan, E., & Kopell, N. (2004). Mechanism and circuitry for clustering and fine discrimination of odors in insects. Proceedings of the National Academy of Sciences of the United States of America, 101, 17861–17866. doi:10.1073/pnas.0407858101.
Sivan, E., & Kopell, N. (2006). Oscillations and Slow Patterning in the Antennal Lobe. Journal of Computational Neuroscience, 20, 85–96. doi:10.1007/s10827-006-4087-z.
Sloper, J., & Powell, T. (1978). Ultrastructural features of the sensori-motor cortex of the primate. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 285, 124–139.
Stopfer, M., Bhagavan, S., Smith, B., & Laurent, G. (1997). Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature, 390, 70–74. doi:10.1038/36335.
Strausfeld, N., Hansen, L., Li, Y., Gomez, R., & Ito, K. (1998). Evolution, discovery, and interpretations of arthropod mushroom bodies. Learning & Memory (Cold Spring Harbor, N.Y.), 5, 11–37.
Treloar, H., Feinstein, P., Mombaerts, P., & Greer, C. (2002). Specificity of glomerular targeting by olfactory sensory axons. The Journal of Neuroscience, 22, 2469–2477.
Vickers, N., & Christensen, T. (1998). A combinatorial model of odor discrimination using a small array of contiguous, chemically defined glomeruli. Annals of the New York Academy of Sciences, 855, 514–516. doi:10.1111/j.1749-6632.1998.tb10617.x.
Vickers, N., Christensen, T., & Hildebrand, J. (1998). Combinatorial odor discrimination in the brain: attractive and antagonistic odor blends are represented in distinct combinations of uniquely identifiable glomeruli. The Journal of Comparative Neurology, 400, 35–36. doi:10.1002/(SICI)1096-9861(19981012) 400:1<35::AID-CNE3>3.0.CO;2-U.
Vosshall, L., Wong, A., & Axel, R. (2000). An olfactory sensory map in the fly brain. Cell, 102, 147–159. doi:10.1016/S0092-8674(00)00021-0.
Wachowiak, M., & Cohen, L. (2001). Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron, 32, 723–735. doi:10.1016/S0896-6273(01)00506-2.
Wang, J., Wong, A., Flores, J., Vosshall, L., & Axel, R. (2003). Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell, 112, 271–282. doi:10.1016/S0092-8674(03)00004-7.
Wehr, M., & Laurent, G. (1999). Relationship between afferent and central temporal patterns in the locust olfactory system. The Journal of Neuroscience, 19, 381–390.
Wilson, R., & Mainen, Z. (2006). Early events in olfactory processing. Annual Review of Neuroscience, 29, 163–201. doi:10.1146/annurev.neuro.29.051605.112950.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor:
T. Sejnowski
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patel, M., Rangan, A.V. & Cai, D. A large-scale model of the locust antennal lobe. J Comput Neurosci 27, 553–567 (2009). https://doi.org/10.1007/s10827-009-0169-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-009-0169-z