Abstract
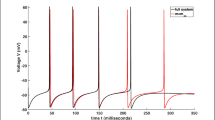
Exposed to a sufficiently high extracellular potassium concentration ([K + ]o), the neuron can fire spontaneous discharges or even become inactivated due to membrane depolarisation (‘depolarisation block’). Since these phenomena likely are related to the maintenance and propagation of seizure discharges, it is of considerable importance to understand the conditions under which excess [K + ]o causes them. To address the putative effect of glial buffering on neuronal activity under elevated [K + ]o conditions, we combined a recently developed dynamical model of glial membrane ion and water transport with a Hodgkin–Huxley type neuron model. In this interconnected glia-neuron model we investigated the effects of natural heterogeneity or pathological changes in glial membrane transporter density by considering a large set of models with different, yet empirically plausible, sets of model parameters. We observed both the high [K + ]o-induced duration of spontaneous neuronal firing and the prevalence of depolarisation block to increase when reducing the magnitudes of the glial transport mechanisms. Further, in some parameter regions an oscillatory bursting spiking pattern due to the dynamical coupling of neurons and glia was observed. Bifurcation analyses of the neuron model and of a simplified version of the neuron-glia model revealed further insights about the underlying mechanism behind these phenomena. The above insights emphasise the importance of combining neuron models with detailed astroglial models when addressing phenomena suspected to be influenced by the astroglia-neuron interaction. To facilitate the use of our neuron-glia model, a CellML version of it is made publicly available.








Similar content being viewed by others
References
Bazhenov, M., Timofeev, I., Steriade, M., & Sejnowski, T. J. (2004). Potassium model for slow (2–3 hz) in vivo neocortical paroxysmal oscillations. Journal of Neurophysiology, 92(2), 1116–1132. doi:10.1152/jn.00529.2003.
Binder, D. K., & Steinhauser, C. (2006). Functional changes in astroglial cells in epilepsy. Glia, 54(5), 358–368. doi:10.1002/glia.20394.
Boussouf, A., Lambert, R. C., & Gaillard, S. (1997). Voltage-dependent Na + -HCO\(_3^-\) cotransporter and Na + /H + exchanger are involved in intracellular pH regulation of cultured mature rat cerebellar oligodendrocytes. Glia, 19(1), 74–84.
Chen, K. C., & Nicholson, C. (2000). Spatial buffering of potassium ions in brain extracellular space. Biophysical Journal, 78(6), 2776–2797. doi:10.1016/S0006-3495(00)76822-6.
Cressman, J. R., Ullah, G., Ziburkus, J., Schiff, S. J., & Barreto, E. (2009). The influence of sodium and potassium dynamics on excitability, seizures, and the stability of persistent states: I. Single neuron dynamics. Journal of Computational Neuroscience, 26(2), 159–170. doi:10.1007/s10827-008-0132-4.
Dronne, M., Boissel, J., & Grenier, E. (2006). A mathematical model of ion movements in grey matter during a stroke. Journal of Theoretical Biology, 240(4), 599–615. doi:10.1016/j.jtbi.2005.10.023.
Dronne, M., Grenier, E., Dumont, T., Hommel, M., & Boissel, J. (2007). Role of astrocytes in grey matter during stroke: A modelling approach. Brain Research, 1138, 231–242. doi:10.1016/j.brainres.2006.12.062.
Ermentrout, B. (2010). http://www.math.pitt.edu/~bard/xpp/xpp.html.
Ermentrout, B., & Terman, D. (2010). Foundations of mathematical neuroscience. Berlin: Springer.
Fertziger, A. P., & Ranck, J. B. (1970). Potassium accumulation in interstitial space during epileptiform seizures. Experimental Neurology, 26(3), 571–585.
Florence, G., Dahlem, M. A., Almeida, A.-C. G., Bassani, J. W. M., & Kurths, J. (2009). The role of extracellular potassium dynamics in the different stages of ictal bursting and spreading depression: A computational study. Journal of Theoretical Biology, 258(2), 219–228. doi:10.1016/j.jtbi.2009.01.032.
Frankenhäuser, B., & Hodgkin, A. L. (1956). The after-effects of impulses in the giant nerve fibres of loligo. The Journal of Physiology, 131(2), 341–376.
Fröhlich, F., & Bazhenov, M. (2006). Coexistence of tonic firing and bursting in cortical neurons. Physical Review E, 74(3), 031922. doi:10.1103/PhysRevE.74.031922.
Fröhlich, F., Bazhenov, M., Iragui-Madoz, V., & Sejnowski, T. J. (2008). Potassium Dynamics in the epileptic cortex: New insights on an old topic. The Neuroscientist, 14(5), 422–433. doi:10.1177/1073858408317955.
Fröhlich, F., Bazhenov, M., Timofeev, I., Steriade, M., & Sejnowski, T. J. (2006). Slow state transitions of sustained neural oscillations by activity-dependent modulation of intrinsic excitability. The Journal of Neuroscience, 26(23), 6153–6162. doi:10.1523/JNEUROSCI.5509-05.2006.
Grisar, T., Guillaume, D., & Delgado-Escueta, A. V. (1992). Contribution of Na + ,K + -ATPase to focal epilepsy: A brief review. Epilepsy Research, 12(2), 141–149.
Hahn, P. J., & Durand, D. M. (2001). Bistability dynamics in simulations of neural activity in high-extracellular-potassium conditions. Journal of Computational Neuroscience, 11(1), 5–18.
Hunter, P., Robbins, P., & Noble, D. (2002). The IUPS human physiome project. Pflügers Archiv: European Journal of Physiology, 445(1), 1–9. doi:10.1007/s00424-002-0890-1.
Jensen, M. S., & Yaari, Y. (1997). Role of intrinsic burst firing, potassium accumulation, and electrical coupling in the elevated potassium model of hippocampal epilepsy. Journal of Neurophysiology, 77(3), 1224–1233.
Johnston, D., & Wu, S. M-S. (2001). Foundations of cellular neurophysiology. Cambridge: MIT Press.
Kager, H., Wadman, W. J., & Somjen, G. G. (2000). Simulated seizures and spreading depression in a neuron model incorporating interstitial space and ion concentrations. Journal of Neurophysiology, 84(1), 495–512.
Kager, H., Wadman, W. J., & Somjen, G. G. (2002). Conditions for the triggering of spreading depression studied with computer simulations. Journal of Neurophysiology, 88(5), 2700–2712. doi:10.1152/jn.00237.2002.
Kager, H., Wadman, W. J., & Somjen, G. G. (2007). Seizure-like afterdischarges simulated in a model neuron. Journal of Computational Neuroscience, 22(2), 105–128. doi:10.1007/s10827-006-0001-y.
Keynes, R. D. (1951). The ionic movements during nervous activity. The Journal of Physiology, 114(1–2), 119–150.
Lauf, P. K., & Adragna, N. C. (2000). K-Cl cotransport: Properties and molecular mechanism. Cellular Physiology and Biochemistry, 10(5–6), 341–354.
Lebovitz, R. M. (1996). Quantitative examination of dynamic interneuronal coupling via single-spike extracellular potassium ion transients. Journal of Theoretical Biology, 180(1), 11–25. doi:10.1006/jtbi.1996.0074.
Lloyd, C., Halstead, M., & Nielsen, P. (2004). CeIIML: Its future, present and past. Progress in Biophysics & Molecular Biology, 85(2–3), 433–450. doi:10.1016/j.pbiomolbio.2004.01.004.
Lux, H. D., Heinemann, U., & Dietzel, I. (1986). Ionic changes and alterations in the size of the extracellular space during epileptic activity. Advances in Neurology, 44, 619–639.
Nadkarni, S., Jung, P., & Levine, H. (2008). Astrocytes optimize the synaptic transmission of information. PLoS Computational Biology, 4(5), e1000088. doi:10.1371/journal.pcbi.1000088.
Newman, E. A. (1991). Sodium-bicarbonate cotransport in retinal Müller (glial) cells of the salamander. The Journal of Neuroscience, 11(12), 3972–3983.
Orkand, R. K., Nicholls, J. G., & Kuffler, S. W. (1966). Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. Journal of Neurophysiology, 29(4), 788–806.
Østby, I., Øyehaug, L., Einevoll, G. T., Nagelhus, E. A., Plahte, E., Zeuthen, T., et al. (2009a). Astrocytic mechanisms explaining neural-activity-induced shrinkage of extraneuronal space. PLoS Computational Biology, 5(1), e1000272. doi:10.1371/journal.pcbi.1000272.
Østby, I., Øyehaug, L., Einevoll, G. T., Ottersen, O. P., & Omholt, S. W. (2009b). Modeling of astrocytic mechanisms explaining neural activity-induced shrinkage of extracellular space and clearance of excess extracellular potassium. Meeting abstract, 2nd INCF Congress of Neuroinformatics, Pilsen, Czech Republic, 6–8 September 2009.
Pangrsic, T., Potokar, M., Haydon, P. G., Zorec, R., & Kreft, M. (2006). Astrocyte swelling leads to membrane unfolding, not membrane insertion. Journal of Neurochemistry, 99(2), 514–523. doi:10.1111/j.1471-4159.2006.04042.x.
Park, E., & Durand, D. M. (2006). Role of potassium lateral diffusion in non-synaptic epilepsy: A computational study. Journal of Theoretical Biology, 238(3), 666–682. doi:10.1016/j.jtbi.2005.06.015.
Postnov, D. E., Ryazanova, L. S., Brazhe, N. A., Brazhe, A. R., Maximov, G. V., Mosekilde, E., et al. (2008). Giant glial cell: New insight through mechanism-based modeling. Journal of Biological Physics, 34(3–4), 441–457. doi:10.1007/s10867-008-9070-7.
Rose, C. R., Kovalchuk, Y., Eilers, J., & Konnerth, A. (1999). Two-photon Na + imaging in spines and fine dendrites of central neurons. Pflügers Archiv: European Journal of Physiology, 439(1–2), 201–207.
Somjen, G. G. (2004). Ions in the brain: Normal function, seizures, and stroke. Oxford: Oxford University Press.
Somjen, G. G., Kager, H., & Wadman, W. J. (2008). Computer simulations of neuron-glia interactions mediated by ion flux. Journal of Computational Neuroscience, 25(2), 349–365. doi:10.1007/s10827-008-0083-9.
Ullah, G., & Schiff, S. J. (2010). Assimilating seizure dynamics. PLoS Computational Biology, 6(5), e1000776. doi:10.1371/journal.pcbi.1000776.
Volman, V., Ben-Jacob, E., & Levine, H. (2007). The astrocyte as a gatekeeper of synaptic information transfer. Neural Computation, 19(2), 303–326. doi:10.1162/neco.2007.19.2.303.
Ziburkus, J., Cressman, J. R., Barreto, E., & Schiff, S. J. (2006). Interneuron and pyramidal cell interplay during in vitro seizure-like events. Journal of Neurophysiology, 95(6), 3948–3954. doi:10.1152/jn.01378.2005.
Zuckermann, E. C., & Glaser, G. H. (1970). Activation of experimental epileptogenic foci: Action of increased K + in extracellular spaces of the brain. Archives of Neurology, 23(4), 358–364.
Acknowledgements
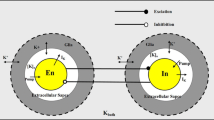
We are grateful for the assistance of Pulasthi Mithraratne in the creation of the diagram in Fig. 1. We are also indebted to John Wyller for helpful discussions, and to Maxim Bazhenov, Giri Krishnan as well as two anonymous reviewers whose comments and suggestions helped improve the quality of the paper. The research has been partially supported by the Research Council of Norway through grants no 178143 and 178892.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: T. Sejnowski
Appendices
Appendix A: Choice of parameters and initial values
Most parameters of the neuron model were set to values equal or in close proximity to values used in Kager et al. (2000) except J NaKATPase,n,max and g leak,K which were estimated by requiring that the net fluxes of sodium and potassium across the neuronal membrane be zero under baseline conditions. Initial values for the gating variables of the neuron model (given in Table 3) were selected as steady-state values of the corresponding model equations.
Initial values for [K + ]n, [K + ]o, [K + ]g, [Na + ]n, [Na + ]o, [Na + ]g, and [HCO\(^-_3\)]o, the glial membrane potential at baseline \(V_{\mathrm{m}}^{\mathrm{(g)}}\), the initial glia volume to area ratio w g and the initial ratio of glial to ECS volume v g/v o = w g/w o, and values for the parameters K m,Na, K m,K, g Na, g Cl, g NKCC1, g NBC and L p were all obtained from available experimental data (see Østby et al. 2009a, for details). Parameters g K, J NaKATPase,g,max, X g and ρ and initial values of ion concentrations [Cl −]g, [Cl −]o, [HCO\(^-_3\)]g for which no empirical estimates were available were derived from the model equations (3a)–(5) to make certain that the model is in a steady-state at baseline conditions.
Since the empirically determined quantities (parameter values and concentrations) come from various studies and different experimental settings, we chose for the simulations reported in Section 4 an approach where the value for each of the selected quantities was randomly drawn from a uniform distribution around the set point value. For these ‘empirically plausible’ parameter sets the values for the baseline ion concentrations were drawn at random from the interval (0.9[S]g,o,1.1[S]g,o), where [S]g,o is the empirical baseline ion concentration of ion S (specified in Table 3). The initial glia volume to area ratio was sampled at random from the interval (1/13 μm, 1/27 μm). The remaining parameters were sampled at random from (0.5P, 1.5P), where P denotes the mean empirical value of any of the above parameters (given in Table 4). Given the randomly sampled values of parameters and initial ion concentrations, values of g K, J NaKATPase,g,max, X g and ρ and initial values of ion concentrations [Cl −]g, [Cl −]o, [HCO\(^-_3\)]g were estimated by, as above, requiring that the model is in a steady-state at baseline conditions.
Appendix B: Model translation
Although mathematical modelling has been identified as a valuable method for analysing large amounts of experimental data, unfortunately, inaccuracies often arise with the current method of mathematical model publication (Hunter et al. 2002; Lloyd et al. 2004). Problems stem from the fact that models are developed and simulated in computer code, but require translation into text and equations for publication in journals. Replicating published results, or further developing a published model, is frequently impeded due to errors introduced during the publishing process such as typographical errors, missing parameters, or equations. Further, even when the model source code is made freely available, the code is often specific to a particular computer platform, or is incompatible with other modelling architectures. CellML is an XML-based modelling language which provides an unambiguous method of defining models of biological processes (Lloyd et al. 2004). It has been developed as a potential solution to the problems associated with publishing and implementing a mathematical model. The current model has been translated into CellML and the code is freely available for download from http://models.cellml.org/e/2e. Model simulations can be run using the Physiome CellML Environment (PCEnv) or Cellular Open Resource (COR), two open source tools which can be downloaded from http://www.cellml.org/downloads/pcenv/ and http://cor.physiol.ox.ac.uk/, respectively.
Rights and permissions
About this article
Cite this article
Øyehaug, L., Østby, I., Lloyd, C.M. et al. Dependence of spontaneous neuronal firing and depolarisation block on astroglial membrane transport mechanisms. J Comput Neurosci 32, 147–165 (2012). https://doi.org/10.1007/s10827-011-0345-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-011-0345-9