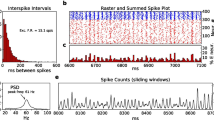
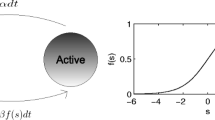
Abstract
Neuronal gamma oscillations have been described in local field potentials of different brain regions of multiple species. Gamma oscillations are thought to reflect rhythmic synaptic activity organized by inhibitory interneurons. While several aspects of gamma rhythmogenesis are relatively well understood, we have much less solid evidence about how gamma oscillations contribute to information processing in neuronal circuits. One popular hypothesis states that a flexible routing of information between distant populations occurs via the control of the phase or coherence between their respective oscillations. Here, we investigate how a mismatch between the frequencies of gamma oscillations from two populations affects their interaction. In particular, we explore a biophysical model of the reciprocal interaction between two cortical areas displaying gamma oscillations at different frequencies, and quantify their phase coherence and communication efficiency. We observed that a moderate excitatory coupling between the two areas leads to a decrease in their frequency detuning, up to ∼6 Hz, with no frequency locking arising between the gamma peaks. Importantly, for similar gamma peak frequencies a zero phase difference emerges for both LFP and MUA despite small axonal delays. For increasing frequency detunings we found a significant decrease in the phase coherence (at non-zero phase lag) between the MUAs but not the LFPs of the two areas. Such difference between LFPs and MUAs behavior is due to the misalignment between the arrival of afferent synaptic currents and the local excitability windows. To test the efficiency of communication we evaluated the success of transferring rate-modulations between the two areas. Our results indicate that once two populations lock their peak frequencies, an optimal phase relation for communication appears. However, the sensitivity of locking to frequency mismatch suggests that only a precise and active control of gamma frequency could enable the selection of communication channels and their directionality.











Similar content being viewed by others
References
Battaglia, D., Witt, A., Wolf, F., Geisel T. (2012). Dynamic effective connectivity of inter-areal brain circuits. PLoS Computational Biology, 8.3, e1002438. doi:10.1371/journal.pcbi.1002438.
Berens, P., Logothetis, N.K., Tolias, A.S. (2012). Visual population codes - towards a common multivariate framework for cell recording and functional imaging. In Local field potentials, BOLD and spiking activity: relationships and physiological mechanisms (pp. 599–624). MIT Press.
Bokil, H., Andrews, P., Kulkarni, J.E., Mehta, S., Mitra, P.P. (2010). Chronux: a platform for analyzing neural signals. Journal of Neuroscience Methods, 192.1, 146–51. doi:10.1016/j.jneumeth.2010.06.020.
Bosman, C.A., Schoffelen, J.-M., Brunet, N., Oostenveld, R., Bastos, A.M., Womelsdorf, T., Rubehn, B., Stieglitz, T., De Weerd, P., Fries, P. (2012). Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron, 75.5, 875–888. doi:10.1016/j.neuron.2012.06.037.
Brunel, N., & Wang, X.-J. (2003). What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. Journal of Neurophysiology, 90.1, 415–430. doi:10.1152/jn.01095.2002.
Buehlmann, A., & Deco, G. (2010). Optimal information transfer in the cortex through synchronization. PLoS Computational Biology, 6(9). doi:10.1371/journal.pcbi.1000934.
Buhl, E.H., Halasy, K., Somogyi, P. (1994). Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature, 368.6474, 823–828. doi:10.1038/368823a0.
Bush, P., & Sejnowski, T. (1996). Inhibition synchronizes sparsely connected cortical neurons within and between columns in realistic network models. Jouranl of Computational Neuroscience, 3.2, 91–110.
Buzsáki, G., Anastassiou, C.A., Koch, C. (2012). The origin of extracellular fields and currents–EEG, ECoG, LFP and spikes. Nature Reviews Neuroscience, 13.6, 407–420.
Buzsáki, G., & Draguhn, A. (2004). Neuronal oscillations in cortical networks. Science, 304.5679, 1926–1929. doi:10.1126/science.1099745.
Buzsáki, G., & Wang, X.-J. (2012). Mechanisms of gamma oscillations. Annual Review of Neuroscience, 35, 203–225. doi:10.1146/annurev-neuro-062111-150444.
Cardin, J.A., Carlén, M., Meletis, K., Knoblich, U., Zhang, F., Deisseroth, K., Tsai, L.-H., Moore, C.I. (2009). Driving fastspiking cells induces gamma rhythm and controls sensory responses. Nature, 459.7247, 663–667. doi:10.1038/nature08002.
Eriksson, D., Vicente, R., Schmidt, K. (2011). A linear model of phase dependent power correlations in neuronal oscillations. Frontiers in Computational Neuroscience, 5(34). doi:10.3389/fncom.2011.00034.
Fisahn, A., Pike, F.G., Buhl, E.H., Paulsen, O. (1998). Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature, 394.6689, 186–189. doi:10.1038/28179.
Fries, P. (2005). A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in Cognitive Sciences, 9.10, 474–480. doi:10.1016/j.tics.2005.08.011.
Garcia-Ojalvo, J., & Sancho, J.M. (1999). Noise in spatially extended systems. New York: Springer-Verlag.
Gruber, T., Müller, M.M., Keil, A., Elbert, T. (1999). Selective visual-spatial attention alters induced gamma band responses in the human EEG. Clinical Neurophysiology, 110.12, 2074–2085.
Gutfreund, Y., Yarom, Y., Segev, I. (1995). Subthreshold oscillations and resonant frequency in guinea-pig cortical neurons: physiology and modelling. Journal of Physiology, 483(Pt 3), 621–640.
Henrie, J.A., & Shapley, R. (2005). LFP power spectra in V1 cortex: the graded effect of stimulus contrast. Journal of Neurophysiology, 94.1, 479–490. doi:10.1152/jn.00919.2004.
Houston, C.M., Bright, D.P., Sivilotti, L.G., Beato, M., Smart, T.G. (2009). Intracellular chloride ions regulate the time course of GABA-mediated inhibitory synaptic transmission. Journal of Neuroscience, 29.33, 10416–10423. doi:10.1523/JNEUROSCI.1670-09.2009.
Kang, Y., Kaneko, T., Ohishi, H., Endo, K., Araki, T. (1994). Spatiotemporally differential inhibition of pyramidal cells in the cat motor cortex. Journal of Neurophysiology, 71.1, 280–293.
Kaplan, E., Purpura, K., Shapley, R.M. (1987). Contrast affects the transmission of visual information through the mammalian lateral geniculate nucleus. Journal of Physiology, 391, 267–288.
Kawaguchi, Y., & Kubota, Y. (1997). GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cerebral Cortex, 7.6, 476–486.
Markram, H., Toledo-Rodriguez, M., Wang, Y., Gupta, A., Silberberg, G., Wu, C. (2004). Interneurons of the neocortical inhibitory system. Nature Reviews Neuroscience, 5.10, 793–807. doi:10.1038/nrn1519.
Mazzoni, A., Panzeri, S., Logothetis, N.K., Brunel, N. (2008). Encoding of naturalistic stimuli by local field potential spectra in networks of excitatory and inhibitory neurons. PLoS Computational Biology, 4.12, e1000239. doi:10.1371/journal.pcbi.1000239.
Mountcastle, V.B. (1998). Perceptual neuroscience: the cerebral cortex. Cambridge: Harvard University Press.
Pikovsky, A., Rosenblum, M., Kurths, J. (2001). Synchronization. A universal concept in nonlinear sciences. Cambridge University Press.
Puia, G., Costa, E., Vicini, S. (1994). Functional diversity of GABA activated Cl- currents in Purkinje versus granule neurons in rat cerebellar slices. Neuron, 12.1, 117–126.
Pulvermüller, F., Birbaumer, N., Lutzenberger, W., Mohr, B. (1997). High-frequency brain activity: its possible role in attention, perception and language processing. Progress in Neurobiology, 52.5, 427–445.
Ray, S., & Maunsell, J.H.R. (2010). Differences in gamma frequencies across visual cortex restrict their possible use in computation. Neuron, 67.5, 885–896. doi:10.1016/j.neuron.2010.08.004.
Roberts, M.J., Lowet, E., Brunet, N.M., Ter Wal, M., Tiesinga, P., Fries, P., De Weerd, P. (2013). Robust gamma coherence between macaque V1 and V2 by dynamic frequency matching. Neuron, 78.3, 523–536. doi:10.1016/j.neuron.2013.03.003.
Roepstorff, A., & Lambert, J.D. (1994). Factors contributing to the decay of the stimulus-evoked IPSC in rat hippocampal CA1 neurons. Journal of Neurophysiology, 72.6, 2911–2926.
Sancristóbal, B., Vicente, R., Sancho, J.M., Garcia-Ojalvo, J. (2013). Emergent bimodal firing patterns implement different encoding strategies during gamma-band oscillations. Frontiers in Computational Neuroscience, 7, 18. doi:10.3389/fncom.2013.00018.
Schoffelen, J.-M., Oostenveld, R., Fries, P. (2005). Neuronal coherence as a mechanism of effective corticospinal interaction. Science, 308.5718, 111–113. doi:10.1126/science.1107027.
Shadlen, M.N., & Movshon, J.A. (1999). Synchrony unbound: a critical evaluation of the temporal binding hypothesis. Neuron, 24.1(67–77), 111–125.
Singer, W. (1999). Neuronal synchrony: a versatile code for the definition of relations? Neuron, 24.1(49–65), 111–125.
Tallon-Baudry, C., Bertrand, O., Delpuech, C., Permier, J. (1997). Oscillatory gamma-band (30-70 Hz) activity induced by a visual search task in humans. Journal of Neuroscience, 17.2, 722–734.
Thomson, D.J. (1982). Spectrum estimation and harmonic analysis. In Proceedings of the IEEE (Vol. 70.9, pp. 1055–1096). doi:10.1109/PROC.1982.12433, http://ieeexplore.ieee.org/xpls/abs∖_all.jsp?arnumber=1456701.
Vicente, R., Gollo, L.L., Mirasso, C.R., Fischer, I., Pipa, G. (2008). Dynamical relaying can yield zero time lag neuronal synchrony despite long conduction delays. Proceedings of the National Academy of Sciences of the United States of America, 105.44, 17157–17162. doi:10.1073/pnas.0809353105.
Voges, N., Guijarro, C., Aertsen, A., Rotter, S. (2010a). Models of cortical networks with long-range patchy projections. Journal of Computational Neuroscience, 28.1, 137–154. doi:10.1007/s10827-009-0193-z.
Voges, N., Schüz, A., Aertsen, A., Rotter, S. (2010b). A modeler’s view on the spatial structure of intrinsic horizontal connectivity in the neocortex. Progress in Neurobiology, 92.3, 277–292. doi:10.1016/j.pneurobio.2010.05.001.
Watts, D.J., & Strogatz, S.H. (1998). Collective dynamics of ’smallworld’ networks. Nature, 393.6684, 440–442. doi:10.1038/30918.
Whittington, M.A., Traub, R.D., Jefferys, J.G. (1995). Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature, 373.6515, 612–615. doi:10.1038/373612a0.
Womelsdorf, T., Schoffelen, J.M., Oostenveld, R., Singer, W., Desimone, R., Engel, A.K., Fries, P. (2007). Modulation of neuronal interactions through neuronal synchronization. Science, 316.5831, 1609–1612. doi:10.1126/science.1139597.
Acknowledgments
R.V. thanks Luiz Lana, Jaan Aru, and David Eriksson for fruitful discussions. The authors also thank Pascal Fries for early discussions on the problem of frequency detuning for CTC. This work has been financially supported by the Ministerio de Ciencia e Innovación (project FIS2012-37655). J.G.O. also acknowledges financial support from the ICREA Academia program. R.V. acknowledges financial support from the Hertie Foundation and Estonian Research Council (project BioMedIT SMTAT13061T).
Conflict of interests
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: J. Rinzel
Rights and permissions
About this article
Cite this article
Sancristóbal, B., Vicente, R. & Garcia-Ojalvo, J. Role of frequency mismatch in neuronal communication through coherence. J Comput Neurosci 37, 193–208 (2014). https://doi.org/10.1007/s10827-014-0495-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-014-0495-7