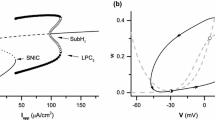
Abstract
The inhibitory restraint necessary to suppress aberrant activity can fail when inhibitory neurons cease to generate action potentials as they enter depolarization block. We investigate possible bifurcation structures that arise at the onset of seizure-like activity resulting from depolarization block in inhibitory neurons. Networks of conductance-based excitatory and inhibitory neurons are simulated to characterize different types of transitions to the seizure state, and a mean field model is developed to verify the generality of the observed phenomena of excitatory-inhibitory dynamics. Specifically, the inhibitory population’s activation function in the Wilson-Cowan model is modified to be non-monotonic to reflect that inhibitory neurons enter depolarization block given strong input. We find that a physiological state and a seizure state can coexist, where the seizure state is characterized by high excitatory and low inhibitory firing rate. Bifurcation analysis of the mean field model reveals that a transition to the seizure state may occur via a saddle-node bifurcation or a homoclinic bifurcation. We explain the hysteresis observed in network simulations using these two bifurcation types. We also demonstrate that extracellular potassium concentration affects the depolarization block threshold; the consequent changes in bifurcation structure enable the network to produce the tonic to clonic phase transition observed in biological epileptic networks.







Similar content being viewed by others
References
Ahmed, O.J., Kramer, M.A., Truccolo, W., Naftulin, J.S., Potter, N.S., Eskandar, E.N., Cosgrove, G.R., Blum, A.S., Hochberg, L.R., & Cash, S.S. (2014). Inhibitory single neuron control of seizures and epileptic traveling waves in humans. BMC Neuroscience, 15(Suppl 1), F3.
Barreto, E., & Cressman, J.R. (2011). Ion concentration dynamics as a mechanism for neuronal bursting. Journal of Biological Physics, 37(3), 361–373.
Ben-Ari, Y. (2002). Excitatory actions of gaba during development: the nature of the nurture. Nature Reviews Neuroscience, 3(9), 728–739.
Beverlin, B., Kakalios, J., Nykamp, D., & Netoff, T.I. (2012). Dynamical changes in neurons during seizures determine tonic to clonic shift. Journal of Computational Neuroscience, 33(1), 41–51.
Buckmaster, P.S., Zhang, G.F., & Yamawaki, R. (2002). Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 22(15), 6650–6658.
Cammarota, M., Losi, G., Chiavegato, A., Zonta, M., & Carmignoto, G. (2013). Fast spiking interneuron control of seizure propagation in a cortical slice model of focal epilepsy. The Journal of Physiology, 591(Pt 4), 807–822.
Dichter, M., & Spencer, W.A. (1969a). Penicillin-induced interictal discharges from the cat hippocampus. I. Characteristics and topographical features. Journal of Neurophysiology, 32(5), 649–662.
Dichter, M., & Spencer, W.A. (1969b). Penicillin-induced interictal discharges from the cat hippocampus. II. Mechanisms underlying origin and restriction. Journal of Neurophysiology, 32(5), 663–687.
Dudek, F.E., & Sutula, T.P. (2007). Epileptogenesis in the dentate gyrus: a critical perspective. Progress in Brain Research, 163(801), 755–773.
Dzhala, V.I., Kuchibhotla, K.V., Glykys, J.C., Kahle, K.T., Swiercz, W.B., Feng, G., Kuner, T., Augustine, G.J., Bacskai, B.J., & Staley, K.J. (2010). Progressive nkcc1-dependent neuronal chloride accumulation during neonatal seizures. The Journal of Neuroscience, 30(35), 11745–11761.
Ellender, T.J., Raimondo, J.V., Irkle, A., Lamsa, K.P., & Akerman, C.J. (2014). Excitatory effects of parvalbumin-expressing interneurons maintain hippocampal epileptiform activity via synchronous afterdischarges. The Journal of Neuroscience, 34(46), 15208– 15222.
Fröhlich, F., Bazhenov, M., Timofeev, I., Steriade, M., & Sejnowski, T.J. (2006). Slow state transitions of sustained neural oscillations by activity-dependent modulation of intrinsic excitability. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(23), 6153–6162.
Fröhlich, F., Sejnowski, T.J., & Bazhenov, M. (2010). Network bistability mediates spontaneous transitions between normal and pathological brain states. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(32), 10734–10743.
Fujiwara-Tsukamoto, Y., Isomura, Y., Imanishi, M., Ninomiya, T., Tsukada, M., Yanagawa, Y., Fukai, T., & Takada, M. (2010). Prototypic seizure activity driven by mature hippocampal fast-spiking interneurons. The Journal of Neuroscience, 30(41), 13679–13689.
Gastaut, H., & Broughton, R.J. (1972). Epileptic seizures: clinical and electrographic features, diagnosis and treatment. Springfield: Thomas. https://books.google.de/books/about/Epileptic_Seizures.html?id=Xb1rAAAAMAAJ&pgis=1.
Goodman, D.F., & Brette, R. (2008). The brian simulator. Frontiers in Neuroscience, 3, 26.
Gulyás, A.I., Megías, M., Emri, Z., & Freund, T.F. (1999). Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 19(22), 10082– 10097.
Heinemann, U., & Lux, H.D. (1977). Ceiling of stimulus induced rises in extracellular potassium concentration in the cerebral cortex of cat. Brain Research, 120(2), 231–249.
Jensen, M.S., & Yaari, Y. (1997). Role of intrinsic burst firing, potassium accumulation, and electrical coupling in the elevated potassium model of hippocampal epilepsy. Journal of Neurophysiology, 77(3), 1224–1233.
Jensen, M.S., Azouz, R., & Yaari, Y. (1994). Variant firing patterns in rat hippocampal pyramidal cells modulated by extracellular potassium. Journal of Neurophysiology, 71(3), 831–839.
Kager, H., Wadman, W.J., & Somjen, G.G. (2007). Seizure-like afterdischarges simulated in a model neuron. Journal of Computational Neuroscience, 22(2), 105–128.
Karlócai, M.R., Kohus, Z., Káli, S., Ulbert, I., Szabó, G., Máté, Z., Freund, T.F., & Gulyás, A.I. (2014). Physiological sharp wave-ripples and interictal events in vitro: what’s the difference? Brain: A Journal of Neurology, 137(Pt 2), 463–485.
Korn, S.J., Giacchino, J.L., Chamberlin, N.L., & Dingledine, R. (1987). Epileptiform burst activity induced by potassium in the hippocampus and its regulation by GABA-mediated inhibition. Journal of Neurophysiology, 57(1), 325–340.
LeBeau, F.E.N., Towers, S.K., Traub, R.D., Whittington, M.A., & Buhl, E.H. (2002). Fast network oscillations induced by potassium transients in the rat hippocampus in vitro. The Journal of Physiology, 542(Pt 1), 167–179.
Megías, M., Emri, Z., Freund, T.F., & Gulyás, A.I. (2001). Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience, 102(3), 527–540.
Meijer, H.G.E., Eissa, T.L., Kiewiet, B., Neuman, J.F., Schevon, C.A., Emerson, R.G., Goodman, R.R., McKhann, G.M., Marcuccilli, C.J., Tryba, A.K., Cowan, J.D., van Gils, S.A., & van Drongelen, W. (2015). Modeling focal epileptic activity in the Wilson-cowan model with depolarization block. Journal of Mathematical Neuroscience, 5, 7.
Moody, W.J., Futamachi, K.J., & Prince, D.A. (1974). Extracellular potassium activity during epileptogenesis. Experimental Neurology, 42(2), 248–263.
Morris, C., & Lecar, H. (1981). Voltage oscillations in the barnacle giant muscle fiber. Biophysical Journal, 35(1), 193–213.
Øyehaug, L., Østby, I., Lloyd, C.M., Omholt, S.W., & Einevoll, G.T. (2012). Dependence of spontaneous neuronal firing and depolarisation block on astroglial membrane transport mechanisms. Journal of Computational Neuroscience, 32(1), 147–165.
Prince, D.A., & Wilder, B.J. (1967). Control mechanisms in cortical epileptogenic foci. “Surround” inhibition. Archives of Neurology, 16(2), 194–202.
Quian Quiroga, R., Blanco, S., Rosso, O.A., Garcia, H., & Rabinowicz, A. (1997). Searching for hidden information with Gabor Transform in generalized tonic-clonic seizures. Electroencephalography and Clinical Neurophysiology, 103(4), 434–439.
Rinzel, J., & Ermentrout, G.B. (1989). Analysis of neural excitability and oscillations. In C.I. Koch, & E. Segev (Eds.), Methods in neuronal modeling (pp. 135–169). Cambridge: MIT Press. http://dl.acm.org/citation.cfm?id=94605.94613.
Scharfman, H.E., Sollas, A.L., Berger, R.E., & Goodman, J.H. (2003). Electrophysiological evidence of monosynaptic excitatory transmission between granule cells after seizure-induced mossy fiber sprouting. Journal of Neurophysiology, 90(4), 2536– 2547.
Schevon, C.A., Weiss, S.A., McKhann, G., Goodman, R.R., Yuste, R., Emerson, R.G., & Trevelyan, A.J. (2012). Evidence of an inhibitory restraint of seizure activity in humans. Nature Communications, 3, 1060.
Schwartz, T.H., & Bonhoeffer, T. (2001). In vivo optical mapping of epileptic foci and surround inhibition in ferret cerebral cortex. Nature Medicine, 7(9), 1063–107.
Shin, D.S.H., Yu, W., Fawcett, A., & Carlen, P.L. (2010). Characterizing the persistent CA3 interneuronal spiking activity in elevated extracellular potassium in the young rat hippocampus. Brain Research, 1331, 39–50.
Somjen, G.G., & Giacchino, J.L. (1985). Potassium and calcium concentrations in interstitial fluid of hippocampal formation during paroxysmal responses. Journal of Neurophysiology, 53(4), 1098–1108.
Sypert, G., & Ward, A. (1974). Changes in extracellular potassium activity during neocortical propagated seizures. Experimental Neurology, 45(1), 19–41.
Thompson, S.M., & Gahwiler, B.H. (1989). Activity-dependent disinhibition. ii. effects of extracellular potassium, furosemide, and membrane potential on ecl-in hippocampal ca3 neurons. Journal of Neurophysiology, 61(3), 512–523.
Toyoda, I., Fujita, S., Thamattoor, A.K., & Buckmaster, P.S. (2015). Unit activity of hippocampal interneurons before spontaneous seizures in an animal model of temporal lobe epilepsy. Journal of Neuroscience, 35 (16), 6600–6618.
Traynelis, S.F., & Dingledine, R. (1988). Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. Journal of Neurophysiology, 59(1), 259–276.
Trevelyan, A.J., & Schevon, C.A. (2013). How inhibition influences seizure propagation. Neuropharmacology, 69, 45–54.
Trevelyan, A.J., Sussillo, D., Watson, B.O., & Yuste, R. (2006). Modular propagation of epileptiform activity: evidence for an inhibitory veto in neocortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(48), 12447–12455.
Trevelyan, A.J., Sussillo, D., & Yuste, R. (2007). Feedforward inhibition contributes to the control of epileptiform propagation speed. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(13), 3383–3387.
Wilson, H.R., & Cowan, J.D. (1972). Excitatory and inhibitory interactions in localized populations of model neurons. Biophysical Journal, 12(1), 1–24.
Wuarin, J.P., & Dudek, F.E. (2001). Excitatory synaptic input to granule cells increases with time after kainate treatment. Journal of Neurophysiology, 85(3), 1067–1077.
Yaari, Y., Konnerth, A., & Heinemann, U. (1986). Nonsynaptic epileptogenesis in the mammalian hippocampus in vitro. II. Role of extracellular potassium. Journal of Neurophysiology, 56(2), 424–438.
Yi, F., DeCan, E., Stoll, K., Marceau, E., Deisseroth, K., & Lawrence, J.J. (2015). Muscarinic excitation of parvalbumin-positive interneurons contributes to the severity of pilocarpine-induced seizures. Epilepsia, 56(2), 297–309.
Zandt, B.J., Visser, S., van Putten, M.J.A.M., & Ten Haken, B. (2014). A neural mass model based on single cell dynamics to model pathophysiology. Journal of Computational Neuroscience.
Ziburkus, J., Cressman, J.R., Barreto, E., & Schiff, S.J. (2006). Interneuron and pyramidal cell interplay during in vitro seizure-like events. Journal of Neurophysiology, 95(6), 3948–3954.
Acknowledgements
This research was supported by the National Science Foundation grant DMS-0847749. We thank Tay Netoff for insightful discussions and motivating our investigation. CMK thanks Bernstein Center Freiburg for their support where part of this work was conducted.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Action Editor: Steven J. Schiff
Rights and permissions
About this article
Cite this article
Kim, C.M., Nykamp, D.Q. The influence of depolarization block on seizure-like activity in networks of excitatory and inhibitory neurons. J Comput Neurosci 43, 65–79 (2017). https://doi.org/10.1007/s10827-017-0647-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-017-0647-7