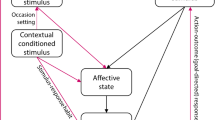
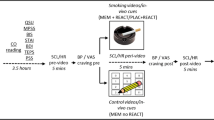
Abstract
Reconsolidation refers to memory reprocessing when consolidated memory is being recalled and restored. Importantly, as memory is being recalled, it could further modify past memories. Memory reconsolidation has been identified as a critical role in various types of mental disorders, in particular drug addiction. In this review, we first review earlier studies related to reconsolidation. Secondly, we characterize memory reconsolidation processing in human brain via neuroimaging studies. Then we focus on the role of reconsolidation and reconsolidation-based interventions in drug addiction. Finally, we highlight the potentials of combining reconsolidation-based interventions and neuroimaging techniques as a therapeutic tool in drug addiction.
Similar content being viewed by others
References
Kandel E R, Dudai Y, Mayford M R. The molecular and systems biology of memory. Cell, 2014, 157: 163–186
Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci, 2009, 10: 224–234
Elsey J W B, van Ast V A, Kindt M. Human memory reconsolidation: a guiding framework and critical review of the evidence. Psychol Bull, 2018, 144: 797–848
Lee J L C, Nader K, Schiller D. An update on memory reconsolidation updating. Trends Cogn Sci, 2017, 21: 531–545
Sandrini M, Cohen L G, Censor N. Modulating reconsolidation: a link to causal systems-level dynamics of human memories. Trends Cogn Sci, 2015, 19: 475–482
Sorg B A. Reconsolidation of drug memories. Neurosci Biobehaval Rev, 2012, 36: 1400–1417
Cadet J L, Bisagno V, Milroy C M. Neuropathology of substance use disorders. Acta Neuropathol, 2014, 127: 91–107
Monfils M H, Holmes E A. Memory boundaries: opening a window inspired by reconsolidation to treat anxiety, trauma-related, and addiction disorders. Lancet Psychiatry, 2018, 5: 1032–1042
Schwabe L, Nader K, Pruessner J C. Reconsolidation of human memory: brain mechanisms and clinical relevance. Biol Psychiatry, 2014, 76: 274–280
Xue Y X, Luo Y X, Wu P, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science, 2012, 336: 241–245
Germeroth L J, Carpenter M J, Baker N L, et al. Effect of a brief memory updating intervention on smoking behavior: a randomized clinical trial. JAMA Psychiatry, 2017, 74: 214–223
Dudai Y, Karni A, Born J. The consolidation and transformation of memory. Neuron, 2015, 88: 20–32
Misanin J R, Miller R R, Lewis D J. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science, 1968, 160: 554–555
Schneider A M, Sherman W. Amnesia: a function of the temporal relation of footshock to electroconvulsive shock. Science, 1968, 159: 219–221
Lewis D J, Bregman N J. Source of cues for cue-dependent amnesia in rats. J Comp Physiol Psychol, 1973, 85: 421–426
Lewis D J, Bregman N J, Mahan J J. Cue-dependent amnesia in rats. J Comp Physiol Psychol, 1972, 81: 243–247
Nader K, Schafe G E, Le Doux J E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature, 2000, 406: 722–726
Debiec J, LeDoux J E, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron, 2002, 36: 527–538
Rose J K, Rankin C H. Blocking memory reconsolidation reverses memory-associated changes in glutamate receptor expression. J Neurosci, 2006, 26: 11582–11587
Schiller D, Monfils M H, Raio C M, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature, 2010, 463: 49–53
Walker M P, Brakefield T, Hobson J A, et al. Dissociable stages of human memory consolidation and reconsolidation. Nature, 2003, 425: 616–620
Ogawa S, Tank D W, Menon R, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA, 1992, 89: 5951–5955
Goense J, Merkle H, Logothetis N K. High-resolution fMRI reveals laminar differences in neurovascular coupling between positive and negative BOLD responses. Neuron, 2012, 76: 629–639
Agren T, Engman J, Frick A, et al. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science, 2012, 337: 1550–1552
Björkstrand J, Agren T, Åhs F, et al. Think twice, it’s all right: long lasting effects of disrupted reconsolidation on brain and behavior in human long-term fear. Behavioural Brain Res, 2017, 324: 125–129
Feng P, Zheng Y, Feng T. Spontaneous brain activity following fear reminder of fear conditioning by using resting-state functional MRI. Sci Rep, 2015, 5: 16701–16711
Han J H, Kushner S A, Yiu A P, et al. Selective erasure of a fear memory. Science, 2009, 323: 1492–1496
Monfils M H, Cowansage K K, Klann E, et al. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science, 2009, 324: 951–955
Cheng D T, Richards J, Helmstetter F J. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learn Mem, 2007, 14: 485–490
Knight D C, Nguyen H T, Bandettini P A. The role of the human amygdala in the production of conditioned fear responses. Neuroimage, 2005, 26: 1193–1200
Schwabe L, Nader K, Wolf O T, et al. Neural signature of reconsolidation impairments by propranolol in humans. Biol Psychiatry, 2012, 71: 380–386
Mahabir M, Tucholka A, Shin L M, et al. Emotional face processing in post-traumatic stress disorder after reconsolidation impairment using propranolol: a pilot fMRI study. J Anxiety Disorders, 2015, 36: 127–133
Richardson M P, Strange B A, Dolan R J. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci, 2004, 7: 278–285
Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci, 2009, 12: 256–258
Przybyslawski J, Roullet P, Sara S J. Attenuation of Emotional and Nonemotional Memories after their Reactivation: Role of β Adrenergic Receptors. J Neurosci, 1999, 19: 6623–6628
Shin L M, Wright C I, Cannistraro P A, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry, 2005, 62: 273–281
Schiller D, Kanen J W, LeDoux J E, et al. Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proc Natl Acad Sci USA, 2013, 110: 20040–20045
Feng P, Zheng Y, Feng T. Resting-state functional connectivity between amygdala and the ventromedial prefrontal cortex following fear reminder predicts fear extinction. Soc Cogn Affect Neurosci, 2016, 11: 991–1001
Bouton M E. Context and behavioral processes in extinction. Learn Memory, 2004, 11: 485–494
Khalaf O, Resch S, Dixsaut L, et al. Reactivation of recall-induced neurons contributes to remote fear memory attenuation. Science, 2018, 360: 1239–1242
Visser R M, Lau-Zhu A, Henson R N, et al. Multiple memory systems, multiple time points: how science can inform treatment to control the expression of unwanted emotional memories. Philos Trans R Soc Lond B Biol Sci, 2018, 373: 1742
Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Curr Opin Neurobiol, 2012, 22: 717–723
Milad M R, Wright C I, Orr S P, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry, 2007, 62: 446–454
Sotres-Bayon F, Quirk G J. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol, 2010, 20: 231–235
Dayan E, Censor N, Buch E R, et al. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci, 2013, 16: 838–844
Sandrini M, Caronni A, Corbo M. Modulating reconsolidation with non-invasive brain stimulation-where we stand and future directions. Front Psychol, 2018, 9: 1430
Grossman N, Bono D, Dedic N, et al. Noninvasive deep brain stimulation via temporally interfering electric fields. Cell, 2017, 169: 1029–1041
Sandrini M, Censor N, Mishoe J, et al. Causal role of prefrontal cortex in strengthening of episodic memories through reconsolidation. Curr Biol, 2013, 23: 2181–2184
Censor N, Dayan E, Cohen L G. Cortico-subcortical neuronal circuitry associated with reconsolidation of human procedural memories. Cortex, 2014, 58: 281–288
Manenti R, Cotelli M, Robertson I H, et al. Transcranial brain stimulation studies of episodic memory in young adults, elderly adults and individuals with memory dysfunction: a review. Brain Stimul, 2012, 5: 103–109
Sandrini M, Cappa S F, Rossi S, et al. The role of prefrontal cortex in verbal episodic memory: rTMS evidence. J Cogn Neurosci, 2003, 15: 855–861
Censor N, Dimyan M A, Cohen L G. Modification of existing human motor memories is enabled by primary cortical processing during memory reactivation. Curr Biol, 2010, 20: 1545–1549
Javadi A H, Cheng P. Transcranial direct current stimulation (tDCS) enhances reconsolidation of long-term memory. Brain Stimul, 2013, 6: 668–674
Sandrini M, Brambilla M, Manenti R, et al. Noninvasive stimulation of prefrontal cortex strengthens existing episodic memories and reduces forgetting in the elderly. Front Aging Neurosci, 2014, 6: 289
Gagnon G, Blanchet S, Grondin S, et al. Paired-pulse transcranial magnetic stimulation over the dorsolateral prefrontal cortex interferes with episodic encoding and retrieval for both verbal and non-verbal materials. Brain Res, 2010, 1344: 148–158
Gagnon G, Schneider C, Grondin S, et al. Enhancement of episodic memory in young and healthy adults: a paired-pulse TMS study on encoding and retrieval performance. Neurosci Lett, 2011, 488: 138–142
Javadi A H, Walsh V. Transcranial direct current stimulation (tDCS) of the left dorsolateral prefrontal cortex modulates declarative memory. Brain Stimul, 2012, 5: 231–241
Drevets W C, Gautier C, Price J C, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry, 2001, 49: 81–96
Hellemans K G C, Everitt B J, Lee J L C. Disrupting reconsolidation of conditioned withdrawal memories in the basolateral amygdala reduces suppression of heroin seeking in rats. J Neurosci, 2006, 26: 12694–12699
Kenny P J, Chen S A, Kitamura O, et al. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci, 2006, 26: 5894–5900
Koob G F, Volkow N D. Neurocircuitry of addiction. Neuropsychopharmacol, 2010, 35: 217–238
Robinson T E, Berridge K C. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev, 1993, 18: 247–291
Robinson T E, Berridge K C. Incentive-sensitization and addiction. Addiction, 2001, 96: 103–114
Chiu C Q, Puente N, Grandes P, et al. Dopaminergic modulation of endocannabinoid-mediated plasticity at GABAergic synapses in the prefrontal cortex. J Neurosci, 2010, 30: 7236–7248
Wang W, Dever D, Lowe J, et al. Regulation of prefrontal excitatory neurotransmission by dopamine in the nucleus accumbens core. J Physiol, 2012, 590: 3743–3769
Milton A L, Everitt B J. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci, 2010, 31: 2308–2319
Tronson N C, Taylor J R. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci, 2007, 8: 262–275
Rodriguez W A, Rodriguez S B, Phillips M Y, et al. Post-reactivation cocaine administration facilitates later acquisition of an avoidance response in rats. Behavioural Brain Res, 1993, 59: 125–129
Fan H Y, Cherng C G, Yang F Y, et al. Systemic treatment with protein synthesis inhibitors attenuates the expression of cocaine memory. Behavioural Brain Res, 2010, 208: 522–527
Alaghband Y, O’Dell S J, Azarnia S, et al. Retrieval-induced NMDA receptor-dependent Arc expression in two models of cocaine-cue memory. Neurobiol Learn Memory, 2014, 116: 79–89
Lv X F, Sun L L, Cui C L, et al. NAc shell Arc/Arg3.1 protein mediates reconsolidation of morphine CPP by increased GluR1 cell surface expression: activation of ERK-coupled CREB is required. Int J Neuropsychopharmacol, 2015, 18: pyv030
Pavlov P I. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Ann Neurosci, 2010, 17: 136–141
Finnie P S B, Nader K. The role of metaplasticity mechanisms in regulating memory destabilization and reconsolidation. Neurosci Biobehaval Rev, 2012, 36: 1667–1707
Merlo E, Bekinschtein P, Jonkman S, et al. Molecular mechanisms of memory consolidation, reconsolidation, and persistence. Neural Plast, 2015, 2015: 687175
Addis D R, Wong A T, Schacter D L. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia, 2007, 45: 1363–1377
Wirkner J, Low A, Hamm A O, et al. New learning following reactivation in the human brain: targeting emotional memories through rapid serial visual presentation. Neurobiol Learn Memory, 2015, 119: 63–68
Forcato C, Bavassi L, de Pino G, et al. Differential left hippocampal activation during retrieval with different types of reminders: an fMRI study of the reconsolidation process. PLoS One, 2016, 11: e0151381
Costanzi M, Cannas S, Saraulli D, et al. Extinction after retrieval: effects on the associative and nonassociative components of remote contextual fear memory. Learn Memory, 2011, 18: 508–518
Ishii D, Matsuzawa D, Matsuda S, et al. No erasure effect of retrieval-extinction trial on fear memory in the hippocampus-independent and dependent paradigms. Neurosci Lett, 2012, 523: 76–81
Jones C E, Ringuet S, Monfils M H. Learned together, extinguished apart: reducing fear to complex stimuli. Learn Memory, 2013, 20: 674–685
Hu J C, Wang W Q, Homan P, et al. Reminder duration determines threat memory modification in humans. Sci Rep, 2018, 8: 8848
Isserles M, Shalev A Y, Roth Y, et al. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder—a pilot study. Brain Stimul, 2013, 6: 377–383
Kaneta T. PET and SPECT imaging of the brain: a review on the current status of nuclear medicine in Japan. Jpn J Radiol, 2020, 38: 343–357
Pan Y, Borragán G, Peigneux P. Applications of functional near-infrared spectroscopy in fatigue, sleep deprivation, and social cognition. Brain Topogr, 2019, 32: 998–1012
Somer E, Allen J, Brooks J, et al. Theta phase-dependent modulation of perception by concurrent transcranial alternating current stimulation and periodic visual stimulation. J Cognitive Neurosci, 2020, 3: 1–11
Song P, Han T, Lin H, et al. Transcranial near-infrared stimulation may increase cortical excitability recorded in humans. Brain Res Bull, 2020, 155: 155–158
Acknowledgements
This work was supported by National Key Basic Research Program (Grant Nos. 2016YFA0400900, 2018YFC0831101), National Natural Science Foundation of China (Grant Nos. 71942003, 31771221, 61773360, 71874170), School Foundation of Anhui Medical University (Grant Nos. 2019xkj016, XJ201907), National Science Foundation for Young Scientists of China (Grant No. 31900766), Major Project of Philosophy and Social Science Research, Ministry of Education of China (Grant No. 19JZD010), Fundamental Research Funds for the Central Universities of China. A portion of the numerical calculations in this study were performed with the supercomputing system at the Supercomputing Centre of USTC.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All authors report no biomedical financial interests or potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Fan, C., Cheng, Y., Gou, H. et al. Neuroimaging and intervening in memory reconsolidation of human drug addiction. Sci. China Inf. Sci. 63, 170103 (2020). https://doi.org/10.1007/s11432-019-2847-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11432-019-2847-8