Abstract
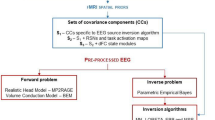
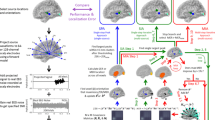
Although fMRI constrained EEG source imaging could be a promising approach to enhancing both spatial and temporal resolutions of independent fMRI and EEG analyses, it has been frequently reported that a hard fMRI constraint may cause severe distortion or elimination of significant EEG sources when there are distinct mismatches between fMRI activations and EEG sources. If estimating actual EEG source locations is important and fMRI prior information is used as an auxiliary tool to enhance the concentration of widespread EEG source distributions, it is reasonable to weaken the fMRI constraint when significantly mismatched sources exist. The present study demonstrates that the mismatch problem may be partially solved by extending the prior fMRI activation regions based on the conventional source imaging results. A hard fMRI constraint is then applied when there is no distinct mismatch, while a weakened fMRI constraint is applied when there are significant mismatches. A preliminary simulation study assuming different types of mismatches such as fMRI invisible, extra, and discrepancy sources demonstrated that this approach can be a promising option to treat mismatched fMRI activations in fMRI constrained EEG source imaging.










Similar content being viewed by others
References
Ahlfors SP, Simpson GV, Dale AM, Belliveau JW, Liu AK, Korvenoja A, Virtanen J, Huotilainen M, Tootell RBH, Aronen HJ, Ilmoniemi RJ (1999) Spatiotemporal activity of a cortical network for processing visual motion revealed by MEG and fMRI. J Neurophysiol 82:2545–2555
Ahlfors SP, Simpson GV (2004) Geometrical interpretation of fMRI-guided MEG/EEG inverse estimates. NeuroImage 22:323–332
Babiloni F, Babiloni C, Carducci F, Romani GL, Rossini PM, Angelone LM, Cincotti F (2003) Multimodal integration of high-resolution EEG and functional magnetic resonance imaging data: a simulation study. NeuroImage 19:1–15
Baillet S, Riera JJ, Marin G, Mangin JF, Aubert J, Garnero L (2001) Evaluation of inverse methods and head models for EEG source localization using a human skull phantom. Phys Med Biol 46:77–96
Bonmassar G, Schwartz DP, Liu AK, Kwong KK, Dale AM, Belliveau JW (2001) Spatiotemporal brain imaging of visual-evoked activity using interleaved EEG and fMRI recordings. NeuroImage 13:1035–1043
Dale AM, Sereno MI (1993) Improved localization of cortical activity by combining EEG and MEG with MRI surface reconstruction: a linear approach. J Cognit Neurosci 5:162–176
Dale AM, Fischl B, Sereno MI (1999) Cortical Surface-Based Analysis I. Segmentation and Surface Reconstruction. NeuroImage 9:179–194
Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E (2000) Dynamic Statistical Parametric Mapping: Combining fMRI and MEG for High-Resolution Imaging of Cortical Activity. Neuron 26:55–67
Disbrow EA, Slutsky DA, Roberts TPL, Krubitzer LA (2005) Functional MRI at 1.5 tesla: A comparison of the blood oxygenation level-dependent signal and electrophysiology. Proc Natl Acad Sci USA 97:9718–9723
Dhond RP, Marinkovic K, Dale AM, Witzel T, Halgren E (2003) Spatiotemporal maps of past-tense verb inflection. NeuroImage 19:91–100
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055
Fujimaki N, Hayakawa T, Nielsen M, Knösche TR, Miyauchi S (2002) An fMRI-constrained MEG source analysis with procedures for dividing and grouping activation. NeuroImage 17:324–343
Fujimaki N, Hayakawa T, Matani A, Okabe Y (2004) Right-lateralized neural activity during inner speech repeated by cues. Neuroreport 15:2341–2345
George JS, Aine CJ, Mosher JC, Schmidt DM, Ranken DM, Schlitt HA, Wood CC, Lewine JD, Sanders J A, Belliveau JW (1995) Mapping Function in the Human Brain with Magnetoencephlaography, Anatomical Magnetic-Resonance-Imaging, and Functional Magnetic-Resonance-Imaging. J Clin Neurophysiol 12:406–431
Gonzalez Andino SL, Blanke O, Lantz G, Thut G, Grave de Peralta Menendez R (2001) The Use of Functional Constraints for the Neuroelectromagnetic Inverse Problem: Alternatives and Caveats. Int J Bioelectrom 3 (internet journal)
Gorodnistky IF, George JS, Rao BD (1995) Neuromagnetic imaging with FOCUSS: a recursive weighted minimum norm algorithm. Electroenceph Clin Neurophys 95:231–251
Hämäläinen MS, Sarvas J (1989) Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans Biomed Eng 36:165–171
Hansen P 1992 Analysis of discrete ill-posed problems by means of the L-curve. SIAM Rev 34:561–580
Haueisen J, Ramon C, Eiselt M, Brauer H, Nowak H (1997) Influence of tissue resistivities on neuromagnetic fields and electric potentials studied with a finite element model of the head. IEEE Trans Biomed Eng 44:727–735
He B, Musha T, Okamoto Y, Homma S, Nakajima Y, Sato T (1987) Electric dipole tracing in the brain by means of the boundary element method and its accuracy. IEEE Trans Biomed Eng 34:406–414
Im CH, Jung HK, Fujimaki N (2005) fMRI-constrained MEG source imaging and consideration of fMRI invisible sources. Hum Brain Mapp 26:110–118
Im CH, Lee SY (2006) A Technique to consider mismatches between fMRI and EEG/MEG sources for fMRI constrained EEG/MEG source imaging: a preliminary simulation study. Phys Med Biol 51:6005–6021
Kincses WE, Braun C, Kaiser S, Elbert T (1999) Modeling extended sources of event-related potentials using anatomical and physiological constraints. Hum Brain Mapp 8:182–193
Korvenoja A, Huttunen J, Salli E, Pohjonen H, Martinkauppi S, Palva JM, Lauronen L, Virtanen J, Ilmoniemi RJ, Aronen HJ (1999) Activation of multiple cortical areas in response to somatosensory stimulation: Combined magnetoencephalographic and functional magnetic resonance imaging. Hum Brain Mapp 8:13–27
Korvenoja A, Aronen HJ, Ilmoniemi J (2001) Functional MRI as a constraint in multi-dipole models of MEG data. Int J Bioelectrom 3 (internet journal)
Lin FH, Witzel T, Hämäläinen MS, Dale AM, Belliveau JW, Stufflebeam SM (2004) Spectral spatiotemporal imaging of cortical oscillations and interactions in the human brain. NeuroImage 23:582–595
Liu AK, Belliveau JW, Dale AM (1998) Spatiotemporal imaging of human brain activity using functional MRI constrained magnetoencephalography data: Monte Carlo simulations. Proc Natl Acad Sci USA 95:8945–8950
Liu AK, Dale AM, Belliveau JW (2002) Monte Carlo simulation studies of EEG and MEG localization accuracy. Hum Brain Mapp 16:47–62
Liu Z, Ding L, He B (2006) Integration of EEG/MEG with MRI and fMRI in Functional Neuroimaging. IEEE Eng Med Biol Mag 25:46–53
Liu Z, Kecman F, He B (2006) Effects of fMRI-EEG mismatches in cortical current density estimation integrating fMRI and EEG: a simulation study. Clinical Neurophysiology, 117(7):1610–1622
Nunez PL, Srinivasan R (2005) Electric Fields of the Brain : The Neurophysics of EEG, 2nd Ed. Oxford University Press, Oxford
Oostendorp TF, Delbeke J, Stegeman DF (2000) The conductivity of the human skull: results of in vivo and in vitro measurements. IEEE Trans Biomed Eng 47:1487–1492
Opitz B, Mecklinger A, von Cramon DY, Kruggel F (1999) Combining electrophysiological and hemodynamic measures of the auditory oddball. Phychophysiol 36:142–147
Pascual-Marqui RD, Michel CM, Lehmann D (1994) Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol 18:49–65
Phillips C, Mattout J, Rugg MD, Maquet P, Friston KJ (2005) An empirical Baysian solution to the source reconstruction problem in EEG. NeuroImage 24:997–1011
Rothman DL, Sibson NR, Hyder F, Shen J, Behar KL, Shulman RG (1999) In vivo MRS studies of the relationship between glutamine-glutamine neurotransmitter cycle and functional neuroenergetics. Phil Trans R Soc Lond B 354:1165–1167
Sato MA, Yoshioka T, Kajihara S, Toyama K, Goda N, Doya K, Kawato M (2004) Hierarchical Bayesian estimation for the MEG inverse problem. NeuroImage, 23(3):806–826
Sekihara K, Nagarajan SS, Poeppel D, Marantz A, Miyashita Y (2001) Reconstructing spatio-temporal activities of neural sources using an MEG vector beamformer technique. IEEE Trans Biomed Eng 48:760–771
Shattuck DW, Leahy RM (2002) BrainSuite: An automated cortical surface identification tool. Med Image Anal 6:129–142
Torquati K, Pizzella V, Babiloni C, Gratta CD, Penna SD, Ferretti A, Franciotti R, Rossini PM, Romani GL (2005) Nociceptive and non-nociceptive sub-regions in the human secondary somatosensory cortex: An MEG study using fMRI constraints. NeuroImage 26:48–56
Vanni S, Warnking J, Dojat M, Delon-Martin C, Bullier J, Segebarth C (2004) Sequence of pattern onset responses in the human visual areas: an fMRI constrained VEP source analysis. NeuroImage 21:801–817
Vitacco D, Brandeis D, Pascual-Marqui R, Martin E (2002) Correspondence of event-related potential tomography and functional magnetic resonance imaging during language processing. Hum Brain Mapp 17:4–12
Wagner M, Fuchs M, Kastner J (2000) fMRI-constrained dipole fits and current density reconstructions. Proc Biomag2000
Acknowledgments
This work was supported in part by a grant of the Korea Health 21 R&D project, Ministry of Health and Welfare, Korea (02–PJ3-PG6-EV07-0002), and in part by a grant (M103KV010016-06K2201-01610) from the Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology of the Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Im, CH. Dealing with mismatched fMRI activations in fMRI constrained EEG cortical source imaging: a simulation study assuming various mismatch types. Med Bio Eng Comput 45, 79–90 (2007). https://doi.org/10.1007/s11517-006-0142-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-006-0142-1