Abstract
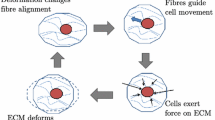
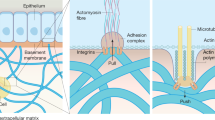
Adherent cells exert contractile forces which play an important role in the spatial organization of the extracellular matrix (ECM). Due to these forces, the substrate experiments a volume reduction leading to a characteristic shape. ECM contraction is a key process in many biological processes such as embryogenesis, morphogenesis and wound healing. However, little is known about the specific parameters that control this process. With this aim, we present a 3D computational model able to predict the contraction process of a hydrogel matrix due to cell–substrate mechanical interaction. It considers cell-generated forces, substrate deformation, ECM density, cellular migration and proliferation. The model also predicts the cellular spatial distribution and concentration needed to reproduce the contraction process and confirms the minimum value of cellular concentration necessary to initiate the process observed experimentally. The obtained continuum formulation has been implemented in a finite element framework. In parallel, in vitro experiments have been performed to obtain the main model parameters and to validate it. The results demonstrate that cellular forces, migration and proliferation are acting simultaneously to display the ECM contraction.






Similar content being viewed by others
References
Ahearne M, Yang Y, El Haj A, Then KY, Liu K (2005) Characterizing the viscoelastic properties of thin hydrogel-based constructs for tissue engineering applications. J R Soc Interface 2:455–463
Askari JA, Buckley PA, Mould AP, Humphries MJ (2009) Linking integrin conformation to function. J Cell Sci 122:167–170
Bell GI (1978) Models for the specific adhesion of cells to cells. Science 200:618–627
Bell E, Ivarsson B, Merrill C (1979) Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA 76:1274–1278
Chiquet M (1999) Regulation of extracellular matrix gene expression by mechanical stress. Mat Biol 18:417–426
Cox TR, Erler JT (2011) Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech 4(2):165–178
Digabel J, Ghibaudo M, Trichet L, Richert A, Ladoux A (2010) Microfabricated substrates as a tool to study cell mechanotransduction. Med Biol Eng Comput 48(10):965–976
Eastwood GL, Trier JS (1974) Epithelial cell proliferation during organogenesis of rat colon. Anat Rec 179:303–309
Elsdale T, Bard J (1972) Collagen substrata for studies on cell behaviour. J Cell Biol 54:626–637
Ferrenq I, Tranqui L, Vailhe B, Gumery PY, Tracqui P (1997) Modelling biological gel contraction by cells: mechanocellular formulation and cell traction force quantification. Acta Biotheor 45:267–293
Filipovic N, Teng Z, Radovic M, Saveljic I, Fotiadis D, Parodi O (2013) Computer simulation of three-dimensional plaque formation and progression in the carotid artery. Med Biol Eng Comput 51(6):607–616
Ghosh K, Pan Z, Guan E, Ge S, Liu Y, Nakamura T, Ren XD, Rafailovich M, Clark RA (2007) Cell adaptation to a physiological relevant ECM mimic with different visco-elastic properties. Biomaterials 28(4):671–679
Guidry C, Grinnell F (1985) Studies on the mechanism of hydrated collagen gel reorganization by human skin fibroblasts. J Cell Sci 79:67–81
Gupton SL, Waterman-Storer CM (2006) Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell 125:1361–1374
Hadjipanayi E, Mudera V, Brown RA (2009) Guiding cell migration in 3D: a collagen matrix with graded directional stiffness. Cell Motil Cytoskelet 66:121–128
Hart S (2012) A primer of collagen biology: synthesis, degradation, subtypes, and role in Dupuytren’s disease. In: Eaton C et al (eds) Dupuytren’s disease and related hyperproliferative disorders. Springer, Berlin, pp 131–142
Hawkins RJ, Piel M, Faure-Andre G, Lennon-Dumenil AM, Joanny JF, Prost J, Voituriez R (2009) Pushing off the walls: a mechanism of cell motility in confinement. Phys Rev Lett 102:58–103
Hibbit K (2002) Abaqus-theory manual. 6.3 Edition
Holmes MJ, Sleeman BD (2000) A mathematical model of tumour angiogenesis incorporating cellular traction and viscoelastic effects. J Theor Biol 202(2):95–112
Hughes T (1987) The finite element method. Prentice-Hall, New Jersey
Kaverina I, Krylyshkina O, Small JV (2002) Regulation of substrate adhesion dynamics during cell motility. Int J Biochem Cell Biol 34:746–761
Lauffenburger D (1989) A simple model for the effects of receptor-mediated cell-substratum adhesion on cell migration. Chem Eng Sci 44(9):1903–1914
Li L, Teng M, Kniss DA, Lasky LC, Yang ST (2001) Effects of filtration seeding on cell density, spatial distribution, and proliferation in nonwoven fibrous matrices. Biotech Prog 17:935–944
Lin S, Yang J, Ho K, Wang C, Yeh C, Huang H (2009) Effects of compressive residual stress on the morphologic changes of fibroblasts. Biol Eng Comput 47(12):1273–1279
Manoussaki D, Lubkin SR, Vernon RB, Murray JD (1996) A mechanical model for the formation of vascular networks in vitro. Acta Biotheor 44:271–282
Manzano S, Poveda-Reyes S, Ferrer GG, Ochoa I, Hamdy Doweidar M (2014) Computational analysis of cartilage implants based on an interpenetrated polymer network for tissue repairing. Comput Methods Program Biomed 116(3):249–259
McKleroy W, Lee TH, Atabai K (2013) Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis. Am J Physiol Lung Cell Mol Physiol 304:709–724
Mousavi SJ, Doweidar MH, Doblaré M (2012) Computational modelling and analysis of mechanical conditions on cell locomotion and cell–cell interaction. Comput Methods Biomech Biomed Eng. doi:10.1080/10255842.2012.710841
Mousavi SJ, Doweidar MH, Doblaré M (2013) 3D computational modelling of cell migration: a mechano-chemo-thermo-electrotaxis approach. J Theor Biol 329:64–73
Mousavi SJ, Doweidar MH, Doblaré M (2013) Cell migration and cell–cell interaction in the presence of mechano-chemo-thermotaxis. Mol Cell Biomech 10(1):1–25
Murray JD (2003) On the mechanochemical theory of biological pattern formation with application to vasculogenesis. C R Biol 326:239–252
Murray JR, Oster GF, Harris AK (1983) A mechanical model for mesenchymal morphogenesis. J Math Biol 17:125–129
Nichols WW, Murphy DG, Cristofalo VJ, Toji LH, Greene AE, Dwight SA (1977) Characterization of a new human diploid cell strain, IMR-90. Science 196(4285):60–63
Odell GM, Oster G, Alberch P, Burnside B (1981) The mechanical basis of morphogenesis: I. Epithelial folding and investigation. Dev Biol 85(2):446–462
Okeyo K, Adachi T, Sunaga J, Hojo M (2009) Actomyosin contractility spatiotemporally regulates actin network dynamics in migrating cells. J Biomech 42:2540–2548
Oster G (1984) On the crawling of cells. J Embryol Exp Morphol 83:329–364
Oster GF, Murray JD, Harris AK (1983) Mechanical aspects of mesenchymal morphogenesis. J Embryol exp Morphol 78:83–135
Quintana L, Muinos TF, Genove E, Olmos M, Borros S, Semino CE (2009) Early tissue patterning recreated by mouse embryonic fibroblasts in a three-dimensional environment. Tissue Eng Part A 15:45–54
Ramtani S (2004) Mechanical modelling of cell-ECM and cell–cell interactions during the contraction of a fibroblast-populated collagen microsphere: theory and model simulation. J Biomech 37:1709–1718
Robert HW (1996) Confocal optical microscopy. Rep Prog Phys 59:427–471
Slack JM, Lin G, Chen Y (2008) Molecular and cellular basis of regeneration and tissue repair. Cell Mol Life Sci 65:54–63
Slaughter BW, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA (2009) Hydrogels in regenerative medicine. Adv Mater 21(32–33):3307–3329
Sohn DH, Lottmann LM, Lum LY, Kim SG, Pedowitz RA, Coutts RD, Sah RL (2002) Effect of gravity on localization of chondrocytes implanted in cartilage defects. Clin Ortho Rel Res 394:254–262
Tabatabai MA, Bursac Z, Eby WM, Singh KP (2011) Mathematical modeling of stem cell proliferation. Med Biol Eng Comput 49(3):253–262
Tingstrom A, Heldin CH, Rubin K (1992) Regulation of fibroblast-mediated collagen gel contraction by platelet-derived growth factor, interleukin-1 alpha and transforming growth factor-beta 1. J Cell Sci 102(Pt 2):315–322
Wartlick O, Mumcu P, Jlicher F, Gonzalez-Gaitan M (2011) Understanding morphogenetic growth control and lessons from flies. Nat Rev Mol Cell Biol 12:594–604
Weinans H, Prendergast PJ (1996) Tissue adaptation as a dynamical process far from equilibrium. Bone 19(2):143–149
Yang Y, Magnay J, Cooling L, Cooper JJ El, Haj J (2004) Effects of substrate characteristics on bone cell response to the mechanical environment. Med Biol Eng Comput 42(1):22–29
Zahm JM, Kaplan H, Herard AL, Doriot F, Pierrot D, Somelette P, Puchelle E (1997) Cell migration and proliferation during the in vitro wound repair of the respiratory epithelium. Cell Motil Cytoskelet 37:33–43
Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P (2006) Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci USA 103:10889–10894
Acknowledgments
The authors would like to thank Berta Naval (Microscopy and Image Service) of the Instituto de Ciencia de Materiales de Aragón, (ICMA) for her technical assistance with the confocal microscope. This study was supported by the grant of Spanish Ministry of Economy and Competitiveness (MINECO MAT2013-46467-C4-3-R and FPU graduate research programme AP2010/2557), and Biomedical Research Networking Center in Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN) initiative. CIBER-BBN is financed by the Instituto de Salud Carlos III with assistance from the European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix: Weak formulation
Appendix: Weak formulation
1.1 Discretization and nonlinear equations system
For the temporal discretization of the governing equations set, we consider the partition \(\mathop \cup \nolimits_{n = 1}^{{n_{\text{step}} - 1}} [t_{n} , t_{n + 1} ]\) of the time interval of interest T and focus on the typical time subinterval \([t_{n} , t_{n + 1} ]\) with \(\Delta t = t_{n + 1} - t_{n} \ge 0\) denoting the corresponding increment of time. It is assumed that the primary unknowns and all derivable quantities are known at time t n . The generalized trapezoidal method is applied [20]. It is defined by \(\alpha \in \left( {0,1} \right]\), such that \(t_{n + \alpha } = t_{n} + \alpha \Delta t\). In this method, the following scheme is used for the temporal discretization of primary variables (only it is shown for c; ρ and u have the same discretization):
where \(c_{n + 1}\), \(\dot{c}_{n + \alpha }\) and \(\dot{c}_{n + 1}\) are approximations of \(c(t_{n + 1} )\), \(\left( {\frac{\partial c}{\partial t}} \right)(t_{n + \alpha } )\) and \(\left( {\frac{\partial c}{\partial t}} \right)(t_{n + 1} )\), respectively. From a practical standpoint, \(\tilde{c}_{n + 1}\) is introduced as a predictor value of \(c_{n + 1}\), which only depends on magnitudes at time t n :
\(\dot{c}_{n + 1}\) can be computed by
Substitution of Eqs. (19) and (20) in the weak form of the problem given by Eq. 16 yields to a semi-discrete set of equations that are discretized in time.
1.2 Spatial discretization of the problem
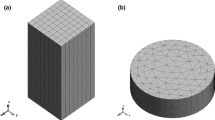
The semi-discrete system is discretized in space using the finite element method. The domain \(\Omega\) is discretized into \(n_{\text{el}}\) elements \(\Omega^{e}\), with \(\Omega = \mathop \cup \nolimits_{e = 1}^{{n_{\text{el}} }} \Omega^{e}\). The primary unknown fields are interpolated within a generic element \(\Omega^{e}\) in terms of the nodal values through shape functions, that is,
where \({\mathbf{c}}^{e}\), \({\varvec{\rho}}^{e}\) and \({\mathbf{u}}^{e}\) are column vectors of nodal values of the primary unknowns at element e and \({\mathbf{N}}_{c}\), \({\mathbf{N}}_{\rho }\) and \({\mathbf{N}}_{{\mathbf{u}}}\) are matrices of element shape functions, that is,
where N i is the shape function associated to element node i and \(n_{\text{en}}\) the number of element nodes. Following a Bubnov–Galerkin scheme, the same shape functions are also applied to interpolate the test functions:
Likewise, the discretization of the related gradients of the test functions and the primary unknowns take the following element-wise format:
The strains are interpolated in the following form:
where \({\mathbf{B}}_{{\mathbf{u}}}\) is a matrix of derivatives of shape functions:
Substituting the Eqs. (21), (23) and (27) into the semi-discrete system and choosing appropriately the arbitrary coefficients \({\delta }{\mathbf{c}}^{e}\), \({\delta }{\varvec{\rho}}^{e}\) and \({\delta }{\mathbf{u}}^{e}\) of the test functions, one can finally arrive to a set of nonlinear algebraic equations which is sufficient to determine the nodal values of the primary unknowns and can be written in the form:
where \({\mathbb{Z}}_{n + 1}\) and \({\tilde{\mathbb{Z}}}_{n + 1}\) are the global column vector of nodal values of the primary unknown fields at time \(t_{n + 1}\) and the corresponding predictor value, respectively. This vector can be obtained as follows:
where \({\mathcal{R}}\) denotes the standard finite element assembly operator and \({\mathbf{d}}_{n + 1}^{e}\) and \({\tilde{\mathbf{d}}}_{n + 1}^{e}\) can be defined by
The internal and external global force vector represented by \({\mathbf{F}}^{\text{int}}\) and \({\mathbf{F}}^{\text{ext}}\) also come from the assembly of element contributions:
where
The element contributions to the internal force can read as:
where \({\mathbf{D}}_{\text{elas}}\) and \({\mathbf{D}}_{\text{visc}}\) are the mechanical behavior matrices:
where \(\xi = \frac{E}{(1 + \nu )(1 - 2\nu )}\) and
In the case of the external force vector, the element contributions have the following expressions:
1.3 Linearization of the problem
The solution of the set equation system 41 is obtained by a standard Newton–Raphson iterative solution procedure. The components of the internal and external global tangent matrices can be defined as the following
Rights and permissions
About this article
Cite this article
Manzano, S., Moreno-Loshuertos, R., Doblaré, M. et al. Structural biology response of a collagen hydrogel synthetic extracellular matrix with embedded human fibroblast: computational and experimental analysis. Med Biol Eng Comput 53, 721–735 (2015). https://doi.org/10.1007/s11517-015-1277-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-015-1277-8