Abstract
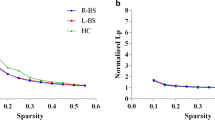
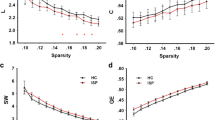
Emerging evidence has revealed widespread stroke-induced brain dysconnectivity, which leads to abnormal network organization. However, there are apparent discrepancies in dysconnectivity between structural connectivity and functional connectivity studies. In this work, resting-state fMRI and structural diffusion tensor imaging were obtained from 26 patients with subacute (10–14 days) intracerebral hemorrhage (ICH) and 20 matched healthy participants (patients/controls = 21/18 after head motion rejection). Graph theoretical approaches were applied to multimodal brain networks to quantitatively compare topological properties between both groups. Prominent small-world properties were found in the structural and functional brain networks of both groups. However, a significant deficit in global integration was revealed in the structural brain networks of the patient group and was associated with more severe clinical manifestations of ICH. Regarding ICH-related nodal deficits, reduced nodal interconnectivity was mainly detected in motor-related regions. Moreover, in the functional brain network, topological properties were mostly comparable between patients with ICH and healthy participants. Beyond the prominent small-world architecture in multimodal brain networks, there are dissociable alterations between structural and functional brain networks in patients with ICH. These findings highlight the potential for using aberrant network metrics as neural biomarkers for evaluation of the severity of ICH.

Intracerebral hemorrhage (ICH) also known as cerebral bleed, a major type of stroke, would significantly affect brain structure and function. Using multimodal neuroimaging, Zhang et al. investigate the ICH-related dysconnectivity in structural and functional brain networks and show a significantly disintegrated structural brain network with a preserved functional network topology in subacute phase (10–14 days).




Similar content being viewed by others
References
Sporns O (2011) The human connectome: a complex network. Ann N Y Acad Sci 1224:109–125
Sun Y, Lim J, Dai Z, Wong K, Taya F, Chen Y, Li J, Thakor N, Bezerianos A (2017) The effects of a mid-task break on the brain connectome in healthy participants: a resting-state functional MRI study. Neuroimage 152:19–30
Watts DJ, Strogatz SH (1998) Collective dynamics of ‘small-world’ networks. Nature 393(6684):440–442
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10(3):186–198
Stam CJ (2014) Modern network science of neurological disorders. Nat Rev Neurosci 15(10):683–695
Grefkes C, Fink GR (2011) Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain 134(Pt 5):1264–1276
Feeney DM, Baron JC (1986) Diaschisis. Stroke 17(5):817–830
Alstott J, Breakspear M, Hagmann P, Cammoun L, Sporns O (2009) Modeling the impact of lesions in the human brain. PLoS Comput Biol 5(6):e1000408
Dijkhuizen RM, van der Marel K, Otte WM, Hoff EI, van der Zijden JP, van der Toorn A, van Meer MP (2012) Functional MRI and diffusion tensor imaging of brain reorganization after experimental stroke. Transl Stroke Res 3:36–43
Wang L, Yu C, Chen H, Qin W, He Y, Fan F, Zhang Y, Wang M, Li K, Zang Y, Woodward TS, Zhu C (2010) Dynamic functional reorganization of the motor execution network after stroke. Brain 133(Pt 4):1224–1238
Cheng L, Wu Z, Sun J, Fu Y, Wang X, Yang GY, Miao F, Tong S (2015) Reorganization of motor execution networks during sub-acute phase after stroke. IEEE Trans Neural Syst Rehabil Eng 23(4):713–723
Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, Khoury J (1996) The ABCs of measuring intracerebral hemorrhage volumes. Stroke 27(8):1304–1305
Gladstone DJ, Danells CJ, Black SE (2002) The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair 16(3):232–240
Shah S, Vanclay F, Cooper B (1989) Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol 42(8):703–709
Sun Y, Dai Z, Li J, Collinson SL, Sim K (2017) Modular-level alterations of structure-function coupling in schizophrenia connectome. Hum Brain Mapp 38(4):2008–2025
Sun Y, Yin Q, Fang R, Yan X, Wang Y, Bezerianos A, Tang H, Miao F, Sun J (2014) Disrupted functional brain connectivity and its association to structural connectivity in amnestic mild cognitive impairment and Alzheimer’s disease. PLoS One 9(5):e96505
Sun Y, Li J, Suckling J, Feng L (2017) Asymmetry of hemispheric network topology reveals dissociable processes between functional and structural brain connectome in community-living elders. Front Aging Neurosci 9:361
Yan CG, Zang YF (2010) DPARSF: a Matlab toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 4:13
Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R (1996) Movement-related effects in fMRI time-series. Magn Reson Med 35(3):346–355
Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP (2013) A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage 76:183–201
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15(1):273–289
Zalesky A, Fornito A, Bullmore E (2012) On the use of correlation as a measure of network connectivity. NeuroImage 60(4):2096–2106
Cui Z, Zhong S, Xu P, He Y, Gong G (2013) PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci 7:42
Mori S, Crain BJ, Chacko VP, van Zijl PC (1999) Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45(2):265–269
Sun Y, Chen Y, Collinson SL, Bezerianos A, Sim K (2017) Reduced hemispheric asymmetry of brain anatomical networks is linked to schizophrenia: a connectome study. Cereb Cortex 27(1):602–615
Buchanan CR, Pernet CR, Gorgolewski KJ, Storkey AJ, Bastin ME (2014) Test-retest reliability of structural brain networks from diffusion MRI. NeuroImage 86:231–243
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52(3):1059–1069
Humphries MD, Gurney K, Prescott TJ (2006) The brainstem reticular formation is a small-world, not scale-freenetwork. Proc Biol Sci 273(1585):503–511
Maslov S, Sneppen K (2002) Specificity and stability in topology of protein networks. Science 296(5569):910–913
Achard S, Bullmore E (2007) Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3(2):e17
Latora V, Marchiori M (2001) Efficient behavior of small-world networks. Phys Rev Lett 87(19):198701
Freeman LC (1977) A set of measures of centrality based on betweenness. Sociometry 40:35–41
van den Heuvel MP, de Lange SC, Zalesky A, Seguin C, Yeo BTT, Schmidt R (2017) Proportional thresholding in resting-state fMRI functional connectivity networks and consequences for patient-control connectome studies: issues and recommendations. NeuroImage 152:437–449
He Y, Wang J, Wang L, Chen ZJ, Yan C, Yang H, Tang H, Zhu C, Gong Q, Zang Y, Evans AC (2009) Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS One 4(4):e5226
Honey CJ, Sporns O (2008) Dynamical consequences of lesions in cortical networks. Hum Brain Mapp 29(7):802–809
Park CH, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, Kim YH (2011) Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke 42(5):1357–1362
Toga AW, Thompson PM (2003) Mapping brain asymmetry. Nat Rev Neurosci 4(1):37–48
Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15(1):1–25
Caliandro P, Vecchio F, Miraglia F, Reale G, Della Marca G, La Torre G, Lacidogna G, Iacovelli C, Padua L, Bramanti P, Rossini PM (2017) Small-world characteristics of cortical connectivity changes in acute stroke. Neurorehabil Neural Repair 31(1):81–94
Bassett DS, Bullmore E (2006) Small-world brain networks. Neuroscientist 12(6):512–523
Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Kust J, Karbe H, Fink GR (2008) Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol 63(2):236–246
Nomura EM, Gratton C, Visser RM, Kayser A, Perez F, D’Esposito M (2010) Double dissociation of two cognitive control networks in patients with focal brain lesions. Proc Natl Acad Sci U S A 107(26):12017–12022
de Vico Fallani F, Astolfi L, Cincotti F, Mattia D, la Rocca D, Maksuti E, Salinari S, Babiloni F, Vegso B, Kozmann G, Nagy Z (2009) Evaluation of the brain network organization from EEG signals: a preliminary evidence in stroke patient. Anat Rec (Hoboken) 292:2023–2031
Dum RP, Strick PL (2002) Motor areas in the frontal lobe of the primate. Physiol Behav 77(4–5):677–682
van den Heuvel MP, Sporns O (2013) Network hubs in the human brain. Trends Cogn Sci 17(12):683–696
Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, Hallett M (2006) Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain 129(Pt 3):791–808
Jones DK, Knosche TR, Turner R (2013) White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. NeuroImage 73:239–254
Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C (2009) Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex 19(3):524–536
Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007) Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage 34(1):144–155
Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann CF, Nichols TE, Ramsey JD, Woolrich MW (2011) Network modelling methods for FMRI. NeuroImage 54(2):875–891
Zalesky A, Fornito A, Harding IH, Cocchi L, Yucel M, Pantelis C, Bullmore ET (2010) Whole-brain anatomical networks: does the choice of nodes matter? NeuroImage 50(3):970–983
Xia M, Wang J, He Y (2013) BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One 8(7):e68910
Acknowledgments
We would like to convey our appreciation to all the participants, especially patients with ICH and their families.
Funding
This work was supported by the General Research Plan B of Zhejiang province (Grant no. 2017KY661 awarded to X. Z.), the ‘Hundred Talents Program’ of Zhejiang University (awarded to Y. S.), the National Natural Science Foundation of China (Grant no. 81801785 awarded to Y. S.), and the Fundamental Research Funds for the Central Universities (Grant no. 2018QNA5017 awarded to Y. S.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The study was approved by the local ethics committee in Shaoxing People’s Hospital, and written informed consent was obtained from each participant (control group) or from the patient’s first degree relatives (patient group).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, X., Yu, X., Bao, Q. et al. Multimodal neuroimaging study reveals dissociable processes between structural and functional networks in patients with subacute intracerebral hemorrhage. Med Biol Eng Comput 57, 1285–1295 (2019). https://doi.org/10.1007/s11517-019-01953-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-019-01953-8