Abstract
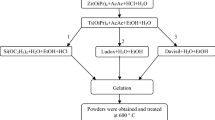
In this study, nanocrystalline ZnO–SnO2 mixed metal oxide powder was prepared by co-precipitation using Zn(CH3COO)2∙2H2O and SnCl4∙5H2O as precursor materials. The powder was characterized by X–ray diffraction, scanning electron microscopy, and Fourier transform infrared spectroscopy. Williamson–Hall method was used to evaluate the micro structural parameters of ZnO–SnO2 such as crystallite sizes and lattice strain. The photoluminescence property of the sample was studied at different temperatures (10–300 K). Results showed that the emission intensity decreases with temperature increasing. The photocatalytic activity at the gas–solid interface was assessed by monitoring the degradation of nitrogen oxides, a major atmospheric pollutant. The results show that the nanocrystalline ZnO–SnO2 mixed metal oxide powder exhibits higher and more stable photocatalytic activity against photocorrosion than ZnO alone.
Graphical abstract










Similar content being viewed by others
References
Marci G, Augugliaro V, López–Muñoz MJ, Martin C, PalmisanoL, Rives V, Schiavello M, Tilley RJ, Venezia AM (2001) Preparation characterization and photocatalytic activity of polycrystalline ZnO/TiO2 systems. 1. Surface and bulk characterization. J Phys Chem B 105(5):1026–1032
Shi L, LiC, GuH FD (2000) Morphology and properties of ultrafine SnO2–TiO2 coupled semiconductor particles. Mater Chem Phys 62(1):62–67
Kant Sharma R, Ghose R (2014) Synthesis of nanocrystalline CuO–ZnO mixed metal oxide powder by a homogeneous precipitation method. Ceram Int l40:10919–10926
Huber F, Venvik H, Ronning M, Walmsley J, Holmen A (2008) Preparation and characterization of nanocrystalline, high-surface area Cu–Ce–Zr mixed oxide catalysts from homogeneous co-precipitation. Chem Eng J 137:686–702
Hwang IS, Kim SJ, Choi JK, Choi J, Ji H, Kim GT, Cao G, Lee JH (2010) Synthesis and gas sensing characteristics of highly crystalline ZnO–SnO2 core–shell nanowires. Sen Act B 148(2):595–600
Ahmad M, Yingying S, Sun H, Shen W, Zhu J (2012) SnO2/ZnO composite structure for the lithium–ion battery electrode. J Solid State Chem 196:326–331
Dharmadasa R, Wijayantha KU, Tahir AA (2010) ZnO–SnO2 composite anodes in extremely thin absorber layer (ETA) solar cells. J Electroanal Chem 646(1):124–132
Zhang M, Sheng G, Fu J, An T, Wang X, Hu X (2005) Novel preparation of nanosizedZnO–SnO2 with high photocatalytic activity by homogeneous co–precipitation method. Mater Lett 59(28):3641–3644
Hoel CA, Mason TO, Gaillard JF, Poeppelmeier KR (2010) Transparent conducting oxides in the ZnO–In2O3–SnO2 system. Chem Mater 22(12):3569–3579
Kumar S, Nigam R, Kundu V, Jaggi N (2015) Sol–gel synthesis of ZnO–SnO2 nanocomposites and their morphological, structural and optical properties. J Mater Sci 26(5):3268–3274
Liu ZQ, Ding LX, Wang ZL, Mao YC, Xie SL, Zhang YM, Lia GR, Tong YX (2012) ZnO/SnO2 hierarchical and flower–like nanostructures: facile synthesis, formation mechanism, and optical and magnetic properties. Cryst Eng Comm 14(6):2289–2295
Chang T, Li Z, Yun G, Jia Y, Yang H (2013) Enhanced photocatalytic activity of ZnO/CuO nanocomposites synthesized by hydrothermal method. Nano–Micro Lett 5(3):163–168
Vijayalakshmi K, Karthick K (2014) High quality ZnO/CuO nanocomposites synthesized by microwave assisted reaction. J Mater Sci 25(2):832–836
Ngamcharussrivichai C, Totarat P, Bunyakiat K (2008) Caand Znmixed oxide as a hetero generous base catalyst fortransesterification of palm kernel oil. Appl Catal A 341:77–85
Zhao X, Zhang F, Xu S, Evans DG, Duan X (2010) From layered double hydroxides to ZnO-based mixed metal oxides by thermal decomposition: transformation mechanism and UV-blocking properties of the product. Chem Mater 22:3933–3942
Li Z, Xiang X, Bai L, Li F (2012) A nanocomposite precursorstrategyto mixed-metal oxide switch excellent catalytic activity for thermal decomposition of ammonium perchlorate. Appl Clay Sci 65–66:14–20
Cui H, Zayat M, Levy D (2005) Sol–gel synthesis of nanoscaled spinelsusing propylene oxide as gelationagent. J Sol–Gel Sci Technol 35:175–181
Zamiri R, Abbastabar Ahangar H, Tobaldi DM, Rebelo A, Seabra MP, Shabani M, Ferreira JMF (2014) Fabricating and characterizing ZnO–ZnS–Ag2S ternary nanostructures with efficient solar–light photocatalytic activity. Phys Chem Chem Phys 16(40):22418–22425
Khorsand Zak A, AbdMajid WH, Abrishami ME, Yousefi R (2011) Solid State Sci 13(1):251–256
Srinivasan G, Kumar RTR, Kumar J (2007) J Sol–Gel Sci Technol 43(2):171–177
Cullity BD (1978) Elements of X-ray Diffractions. Addison-Wesley, Reading, MA
Williamson GK, Hall WH (1953) X–ray line broadening from filed aluminium and wolfram. Acta Metall 1(1):22–31
Scardi P, Leoni M, Delhez R (2004) Line broadening analysis using integral breadth methods: a critical review. J Appl Cryst 37(3):381–390
Barret CS, Massalski TB (1980) Structure of Metals Pergamon Press, Oxford
Tauc J (1968) Optical properties and electronic structure of amorphous Ge and Si. Mater Res Bull 3(1):37–46
Michalow KA, Logvinovich D, Weidenkaff A, Amberg M, Fortunato G, Heel A, Graule T, Rekas M (2009) Synthesis, characterization and electronic structure of nitrogen–doped TiO2nanopowder. Catal Today 144(1):7–12
Caglar Y, Caglar M, Ilican S, Yakuphanoglu F (2009) Determination of the electronic parameters of nanostructure SnO2/p–Si diode. Microelectronic Eng 86(10):2072–2077
Abass AK, Al‐Liabi NA, Taha WA (1988) Optical properties of bromine‐doped SnO2 coatings for solar applications. Physica Status Solidi 106(2):613–618
Dolgonos A, Mason TO, Poeppelmeier KR (2016) Direct optical band gap measurement in polycrystalline semiconductors: a critical look at the Tauc method. J Solid State Chem 240:43–48
Borgwardt M, Wilke M, Kampen T, Mähl S, Xiao M,Spiccia L, Lange KM, Kiyan IY, Aziz EF (2016) Charge transfer dynamics at dye–sensitized ZnO and TiO2 interfaces studied by ultrafast XUV photoelectron spectroscopy. Scientific reports 6:24422–24429
Hamrouni A, Moussa N, Parrino F, Di Paola A, Houas A, Palmisano L (2014) Sol–gel synthesis and photocatalytic activity of ZnO–SnO2 nanocomposites. J Mol Catal A 390:133–141
Rashad MM, Kandil AHT, Ismail AA, Osama I, Ibrahim IA (2014) Photocatalytic decomposition of dyes using ZnO doped SnO2 nanoparticles prepared by solvothermal method. Arabian J Chem 7:71–77
Song C, Dong X (2012) Preparation and Characterization of Tetracomponent ZnO/SiO2/SnO2/TiO2Composite Nanofibers by Electrospinning. Adv Chem Eng Sci 2:108–112
Zamiri R, Zakaria A, Ahanger HA, Darroudi M, Zak AK, Drummen GP (2012) Aqueous starch as a stabilizer in zinc oxide nanoparticle synthesis via laser ablation. J Alloys Compd 516:41–48
Zamiri R, Chenari HM, Moafi HF, Shabani M, Salehizadeh SA, Rebelo A, Kumar JS, Graça MP, Soares MJ, Ferreira JM (2016) Ba–doped ZnO nanostructure: X–ray line analysis and optical properties in visible and low frequency infrared. Ceram Int 42(11):12860–12867
Tobaldi DM, Seabra MP, Otero–Irurueta G, De Miguel YR, Ball RJ, Singh MK, Pullar RC, Labrincha JA (2015) Quantitative XRD characterisation and gas–phase photocatalytic activity testing for visible–light (indoor applications) of KRONO Clean 7000®. RSC Adv 5(124):102911–102918
Ibusuki T, Takeuchi K (1994) Removal of low concentration nitrogen oxides through photoassisted heterogeneous catalysis. J Mol Catal 88(1):93–102
Zhang H, Chen G, Bahnemann DW (2009) Photoelectrocatalytic materials for environmental applications. J Mater Chem 19(29):5089–50121
Xu Y, Schoonen MA (2000) The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am Mineral 85(3–4):543–556
Zhang M, An T, Hu X, Wang C, Sheng G, Fu J (2004) Preparation and photocatalytic properties of a nanometer ZnO–SnO2 coupled oxide. Appl Catal A 260(2):215–222
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Mahmoudi Chenari, H., Zamiri, R., Maria Tobaldi, D. et al. Nanocrystalline ZnO–SnO2 mixed metal oxide powder: microstructural study, optical properties, and photocatalytic activity. J Sol-Gel Sci Technol 84, 274–282 (2017). https://doi.org/10.1007/s10971-017-4484-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-017-4484-y