Abstract
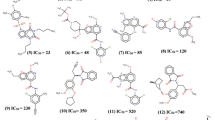
Cyclic nucleotide phosphodiesterases (PDEs) comprise a complex group of enzymes; five major PDE families or classes with distinctive properties have been identified. Among these a great deal of interest has recently been focused on the so called cGMP-inhibited low Km cAMP phosphodiesterase (cGI PDE) or PDE III. A number of positive inotropic agents, including the well-known milrinone, display a specific inhibition of PDE III as primary mechanism of action. Recent studies have been carried out to develop a pharmacophore model of the PDE III active site. We therefore performed molecular modelling and 3D-SAR studies so as to better define structural requirements for potent and selective enzymatic inhibition. The DISCO (DIStance COmparison) strategy has been applied on a set of compounds taken from literature and a milrinone analogue previously synthesized by us, all of which are characterized by a marked inotropic effect but with varying degrees of enzyme selectivity. A common pharmacophoric model was derived, validated and considered as starting point to perform a 3D-SAR study using the GRID force field and PCA (Principal Component Analysis) with the aim of rationally designing more selective inhibitors. This paper presents the results of this theoretical approach.
Similar content being viewed by others
References
Alousi, A.A., Canter, J.M., Montenaro, M.J., Fort, D.J. and Ferrari, R.A., J. Cardiovasc. Pharmacol., 5 (1983) 792.
Butt, E., Beltman, J., Becker, D.E., Jensen, G.S., Rybalkih, S.D., Jastorff, B. and Beavo, J.A., Mol. Pharmacol., 47 (1995) 340.
Bristol, J.A., Sircar, I., Moos, W.H., Evans, D.B. and Weishaar, R.E., J. Med. Chem., 27 (1984) 1099.
Erhardt, P.W., Hagedorn III, A.A. and Sabio, M., Mol. Pharmacol., 33 (1988) 1.
Moos, W.H., Humblet, C.C., Sircar, I., Rithner, C., Weishaar, R.E., Bristol, J.A. and McPhail, A.T., J. Med. Chem., 30 (1987) 1963.
Erhardt, P.W. and Chou, Y.L., Life Sci., 49 (1991) 553.
Mosti, L., Menozzi, G., Schenone, P., Dorigo, P., Gaion, R.M., Benetollo, F. and Bombieri, G., Eur. J. Med. Chem., 24 (1989) 517.
Martin, Y.C., Bures, M.G., Danaher, E.A., De Lazzer, J., Lico, I. and Pavlik, P.A., J. Comput.-Aided Mol. Design, 7 (1993) 83.
Sybyl, Version 6.2, Tripos Inc., St. Louis, MO, 1995.
Goodford, P., J. Chemometrics, 10 (1996) 107.
Manly, B.F., Multivariate Statistical Methods – A Primer, Chapman and Hall, London, 1989.
MacroModel, Version 5.5, Columbia University, New York, NY, 1996.
Mohamadi, F., Richards, N.G.J., Guida, W.C., Liskamp, R., Caufield, C., Chang, G., Hendrickson, T. and Still, W.C., J. Comput.-Aided Mol. Design, 4 (1990) 440.
Gundertofte, K., Liljefors, T., Norrby, P.O. and Pettersson, I., J. Comput. Chem., 17 (1996) 429.
Apaya, R.P., Lucchese, B., Price, S.L. and Vinter, J.G., J. Comput.-Aided Mol. Design, 9 (1995) 33.
Fossa, P., Altomare, C., Cellamare, S., Summo, L., Mosti, L. and Carotti, A., Acta First Italian–Swiss Meeting Med. Chem., (1997) 128.
Allen, F.H., Bellard, S., Brice, M.D., Cartwright, B.A., Doubleday, A., Higgs, H., Hummelink, T., Hummelink-Peters, B.G., Kennard, O., Motherwell, W.D.S., Rodgers, J.R. and Watson, D.G., Acta Crystallogr., B35 (1979) 2331.
Singh, B., Bacon, E.R., Lesher, G.Y., Robinson, S., Pennock, P.O., Bode, D.C., Pagani, E.D., Bentley, R.G., Connell, M.J., Hamel, L.T. and Silver, P.J., J. Med. Chem., 38 (1995) 2546.
Jin, S.L.C., Swinnen, J.V. and Conti, M., J. Biol. Chem., 267 (1992) 18929.
Schudt, C., Winder, S., Müller, B. and Ukena, D., Biochem. Pharmacol., 42 (1991) 153.
Jonas, R., Klockow, M., Lues, I., Prücher, H., Schliep, H.J. and Wurziger, H., Eur. J. Med. Chem., 28 (1993) 129.
Singh, B., Lesher, G.Y., Pluncket, K.C., Pagani, E.D., Bode, D.C., Bentley, R.G., Connell, M.J., Hamel, L.T. and Silver, P.J., J. Med. Chem., 35 (1992) 4858.
Singh, B., Bacon, E.R., Robinson, S., Fritz, R.K., Lesher, G.Y., Kumar, V., Dority, J.A., Reuman, M., Kuo, G.H., Eissenstat, M.A., Pagani, E.D., Bode, D.C., Bentley, R.G., Connell, M.J., Hamel, L.T. and Silver, P.J., J. Med. Chem., 37 (1994) 248.
U.S. Patent 5141931, Annu. Drug Data Report, 17 (1995) 256.
Sugioka, M., Ito, M., Masuoka, H., Ichikawa, K., Konishi, T., Tanaka, T. and Nakano, T., Naunyn-Schmiedeberg's Arch. Pharmacol., 350 (1994) 284.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fossa, P., Boggia, R. & Mosti, L. Toward the identification of the cardiac cGMP inhibited-phosphodiesterase catalytic site. J Comput Aided Mol Des 12, 361 (1998). https://doi.org/10.1023/A:1007928412086
Issue Date:
DOI: https://doi.org/10.1023/A:1007928412086