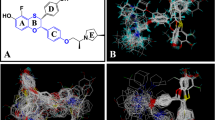
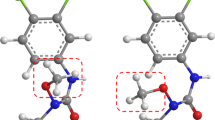
Abstract
One of the major challenges in computational approaches to drug design is the accurate prediction of binding affinity of biomolecules. In the present study several prediction methods for a published set of estrogen receptor ligands are investigated and compared. The binding modes of 30 ligands were determined using the docking program AutoDock and were compared with available X-ray structures of estrogen receptor-ligand complexes. On the basis of the docking results an interaction energy-based model, which uses the information of the whole ligand-receptor complex, was generated. Several parameters were modified in order to analyze their influence onto the correlation between binding affinities and calculated ligand-receptor interaction energies. The highest correlation coefficient (r 2 = 0.617, q 2 LOO = 0.570) was obtained considering protein flexibility during the interaction energy evaluation. The second prediction method uses a combination of receptor-based and 3D quantitative structure-activity relationships (3D QSAR) methods. The ligand alignment obtained from the docking simulations was taken as basis for a comparative field analysis applying the GRID/GOLPE program. Using the interaction field derived with a water probe and applying the smart region definition (SRD) variable selection, a significant and robust model was obtained (r 2 = 0.991, q 2 LOO = 0.921). The predictive ability of the established model was further evaluated by using a test set of six additional compounds. The comparison with the generated interaction energy-based model and with a traditional CoMFA model obtained using a ligand-based alignment (r 2 = 0.951, q 2 LOO = 0.796) indicates that the combination of receptor-based and 3D QSAR methods is able to improve the quality of the underlying model.
Similar content being viewed by others
References
Hansch, C. and Leo, A., Exploring QSAR. Fundamentals and Applications in Chemistry and Biology, American Chemical Society, Washington, DC, 1995.
Kubinyi, H., Drug Discovery Today, 2 (1997) 457.
Cramer III, R.D., Patterson, D.E. and Bunce, J.D., J. Am. Chem. Soc., 110 (1988) 5959.
Kubinyi, H. (Ed.), 3D QSAR in Drug Design. Theory, Methods and Applications, ESCOM, Leiden, 1993.
Kuntz, J.D., Science, 257 (1992) 1078.
Kim, K., J. Comput.-Aided Mol. Design, 7 (1993) 71.
Folkers, G., Merz, A. and Rognan, D., In Kubinyi, H. (Ed.), 3D QSAR in Drug Design. Theory,Methods and Applications, ESCOM, Leiden, 1993, p. 583.
Klebe, G. and Abraham, U., J. Med. Chem., 36 (1993) 70.
Van de Waterbeemd, H., Testa, B. and Folkers, G. (Eds) Computer-Assisted Lead Finding and Optimization. Current Tools for Medicinal Chemistry, Verlag Helvetica Chimica Acta, Basel, Switzerland, 1997.
Höltje, H.-D. and Folkers, G., Molecular Modeling: Basic Principles and Applications, VCH Verlagsgesellschaft, Weinheim, Germany, 1997.
Kramer, B., Rarey, M. and Lengauer, T., Proteins, Suppl. 1, (1997) 221.
Morris, G.M., Goodsell, D.S., Huey, R. and Olson, A.J., J. Comput.-Aided Mol. Design, 8 (1994) 243.
Lybrand, T.P., Curr. Opin. Struct. Biol., 5 (1995) 224.
Meng, E., Shoichet, B.K. and Kuntz. I.D., J. Comput. Chem., 13 (1992) 505.
Tame, J.R.H., J. Comput.-Aided Mol. Design, 13 (1999) 99.
Böhm, H.J., J. Comput.-Aided Mol. Design, 12 (1998) 309.
Böhm, H.J., J. Comput.-Aided Mol. Design, 8 (1994) 243.
Wang, R., Liu, L., Lai, L. and Tang, Y., J. Mol. Model., 4 (1998) 379.
Lemmen, C., Lengauer, T. and Klebe, G., J. Med. Chem., 41 (1998) 4502.
Waller, C.L., Oprea, T.I., Giolitti, A. and Marshall, G.R., J. Med. Chem., 36 (1993) 4152.
De Priest, S.A., Mayer, D., Naylor, C.B. and Marshall, G.R., J. Am. Chem. Soc., 115 (1993) 5372.
Cho, S.J., Garsia, M.L., Bier, J. and Tropsha, A., J. Med. Chem., 39 (1996) 5064.
Vaz, R.J., McLean, L.R. and Pelton, J.T., J. Comput.-Aided Mol. Design, 12 (1998) 99.
Sippl, W., Contreras, J.M., Rival, Y. and Wermuth, C.G., In Gundertofte, K. (Ed.), Molecular Modelling and Prediction of Bioactivity, Plenum Press, New York, NY, in press.
Pastor, M., Cruciani, G. and Watson, K., J. Med. Chem., 40 (1997) 4089.
Sadler, B.R., Cho, S.J., Ishaq, K.S., Chae, K. and Korach, K.S., J. Med. Chem., 41 (1998) 2261.
Brzozowski, A.M., Pike, A.C., Dauter, Z., Hubbard, R.E., Bonn, T., Engstrom, O., Ohman, L., Greene, G.L., Gustafsson, J.A. and Carlquist, M., Nature, 389 (1997) 753.
Shiau, A.K., Barstad, D., Loria, P.M., Cheng, L., Kushner, P.J., Agard, D.A. and Greene, G.L., Cell, 95 (1998) 927.
Bernstein, F.C., Koetzle, T.F., Williams, G.J.B., Meyer, E.F., Brice, M.D., Rodgers, J.R., Kennard, O., Shimanouchi, T. and Tasumi, M., J. Mol. Biol., 112 (1977) 535.
Weiner, S.J., Kollman, P.A., Case, D.A., Singh, U.C., Ghio, C., Alagona, G., Profeta, S. and Weiner, P.J., J. Am. Chem. Soc., 106 (1984) 765.
Singh, U.C. and Kollman, P.A., J. Comput. Chem., 5 (1984) 129.
Vedani, A. and Huhta, D.W., J. Am. Chem. Soc., 112 (1990) 112 269.
Goodsell, D.S., Morris, G.M. and Olson, A.J., J. Mol. Recogn., 9 (1996) 1.
Rao, M.J. and Olson, A.J., Proteins Struct. Funct. Genet., 34 (1999) 173.
Goodford, P.J., J. Med. Chem., 28 (1985) 849.
Vedani, A. and Dunitz, J.D., J. Am. Chem. Soc., 107 (1985) 7653.
PrGen 1.5.6, Biographics Laboratory, Basel, Switzerland.
GRID, v. 16, Molecular Discovery Ltd., Oxford, U.K.
SYBYL 6.5, Tripos Associates, Inc., St. Louis, MO, U.S.A.
Gasteiger, J. and Marsilli, M., Tetrahedron, 36 (1980) 3219.
Spartan 4.1.1, Wavefunction, Irvine, CA, U.S.A.
GOLPE version 4.0, Multivariate Infometric Analysis, Perugia, Italy.
Baroni, M., Constantino, G., Cruciani, G., Riganelli, D., Valigli, R. and Clementi, S., Quant. Struct.-Act. Relat., 12 (1993) 9.
Cruciani, G. and Watson, K., J. Med. Chem., 37 (1994) 2589.
Pastor, M., Cruciani, G. and Clementi, S., J. Med. Chem., 40 (1997) 1455.
Oprea, T.I. and Garcia, A.E., J. Comput.-Aided Mol. Design, 10 (1996) 186.
Tong, W., Perkins, R., Xing, L., Welsh, W.J. and Sheehan, D.M., Endocrinology, 138 (1997) 4022.
Taylor, N.R. and von Itzstein, M., J. Comput.-Aided Mol. Design, 10 (1996) 233.
Jiang, H., Chen, K., Tang, Y., Chen, J., Li, Q., Wang, Q. and Ji, R., J. Med. Chem., 40 (1997) 3085.
Viswanadhan, V.N., Reddy, M.R., Wlodawer, A., Varney, M.D. and Weinstein, J., J. Med. Chem., 39 (1996) 705.
Bursi, R. and Grootenhuis, P.D.J., J. Comput.-Aided Mol. Design, 13 (1999) 221.
Klebe, G., J. Mol. Biol., 237 (1994) 212.
Klebe, G. and Böhm, H., J. Recept. Signal Transduct. Res., 17 (1997) 459.
Waller, C.L., Oprea, T.I., Chae, K., Park, H.K., Korach, K.S., Laws, S.C., Wiese, T.E., Kelce, W.R. and Gray, L.E., Chem. Res. Toxicol., 9 (1996) 1240.
Waller, C.L., Minor, D.L. and McKinney, J.D., Environ. Health Perspect., 103 (1995) 702.
Böhm, M., Stürzebecher, J. and Klebe, G., J. Med. Chem., 42 (1999) 458.
Klebe, G. and Abraham, U., J. Comput.-Aided Mol. Design, 13 (1999) 1.
Kim, K.H., Greco, G. and Novellino, E., Perspect. Drug. Discov. Design, 12 (1998) 257.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sippl, W. Receptor-based 3D QSAR analysis of estrogen receptor ligands – merging the accuracy of receptor-based alignments with the computational efficiency of ligand-based methods. J Comput Aided Mol Des 14, 559–572 (2000). https://doi.org/10.1023/A:1008115913787
Issue Date:
DOI: https://doi.org/10.1023/A:1008115913787