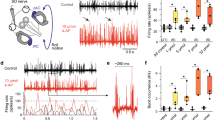
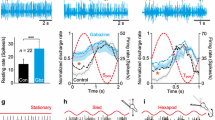
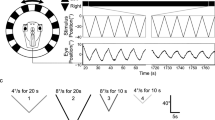
Abstract
Through the process of habituation, continued exposure to low-frequency (0.01 Hz) rotation in the dark produced suppression of the low-frequency response of the vestibulo-ocular reflex (VOR) in goldfish. The response did not decay gradually, as might be expected from an error-driven learning process, but displayed several nonlinear and nonstationary features. They included asymmetrical response suppression, magnitude-dependent suppression for lower- but not higher-magnitude head rotations, and abrupt-onset suppressions suggestive of a switching mechanism. Microinjection of lidocaine into the vestibulocerebellum of habituated goldfish resulted in a temporary dishabituation. This suggests that the vestibulocerebellum mediates habituation, presumably through Purkinje cell inhibition of vestibular nuclei neurons. The habituated VOR data were simulated with a feed-forward, nonlinear neural network model of the VOR in which only Purkinje cell inhibition of vestibular nuclei neurons was varied. The model suggests that Purkinje cell inhibition may switch in to introduce nonstationarities, and cause asymmetry and magnitude-dependency in the VOR to emerge from the essential nonlinearity of vestibular nuclei neurons.
Similar content being viewed by others
References
Achenbach KE, Goodman DC (1968) Cerebellar projections to pons, medulla, and spinal cord in the albino rat. Brain Behav. Evol. 1:43–57.
Allum JHJ, Graf W (1977) Time constants of vestibular nuclei neurons in the goldfish: A model with ocular proprioception. Biol. Cybern. 28:95–99.
Allum JHJ, Graf W, Dichgans J, Schmidt CL (1976) Visual-vestibular interactions in the vestibular nuclei of the goldfish. Exp. Brain Res. 26:463–485.
Anastasio TJ, Robinson DA (1989) The distributed representation of vestibulo-oculomotor signals by brain-stem neurons. Biol. Cybern. 61:79–88.
Angaut P, Brodal A (1967) The projection of the “vestibulocerebellum” onto the vestibular nuclei in the cat. Arch Ital. Biol. 105:441–479.
Angelaki DE, Hess BJM (1994) The cerebellar nodulus and ventral uvula control the torsional vestibulo-ocular reflex. J. Neurophysiol. 72:1443–1447.
Bahill AT, McDonald JD (1983) Frequency limitations and optimal step size for the two-point central difference derivative algorithm with applications to human eye movement data. IEEE Trans. Biomed. Eng. 30:191–194.
Balaban CD (1984) Olivo-vestibular and cerebello-vestibular connections in albino rabbits. Neurosci. 12:129–149.
Buettner UW, Henn V, Young LR (1981) Frequency response of the vestibulo-ocular reflex (VOR) in the monkey. Aviat. Space Environ. Med. 52:73–77.
Butler AB, Hodos W (1996) Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. John Wiley, New York. p. 194.
Carleton SG, Carpenter MB (1983) Afferent and efferent connections of the medial, inferior, and lateral vestibular nuclei in the cat and monkey. Brain Res. 278:29–51.
Carpenter MB, Cowie RJ (1985) Connections and oculomotor projections of the superior vestibular nucleus and cell group “y. ” Brain Res. 336:265–287.
Clément G, Courjon JH, Jeannerod M, Schmid R (1981) Unidirectional habituation of vestibulo-ocular responses by repeated rotational or optokinetic stimulations in the cat. Exp. Brain Res. 42:34–42.
Cohen H, Cohen B, Raphan T, Waespe W (1992a) Habituation and adaptation of the vestibuloocular reflex: A model of differential control by the vestibulocerebellum. Exp. Brain Res. 90:526–538.
Cohen B, Reisine H, Yokota J, Raphan T (1992b) The nucleus of the optic tract: Its function in gaze stabilization and control of visual-vestibular interaction. In: B Cohen, DL Tomko, F Guedry, eds. Sensing and Controlling Motion: Vestibular and Sensorimotor Function. Annals of the New York Academy of Sciences, New York, vol. 656, pp. 277–296.
Collins WE (1964a) Primary, secondary, and caloric nystagmus of the cat following habituation to rotation. J. Comp. Physiol. Psychol. 57:417–421.
Collins WE (1964b) Task-control of arousal and the effects of repeated unidirectional angular acceleration on human vestibular responses. Acta Otolaryngol. Suppl. 190:1–34.
Collins WE, Updegraff BP (1965) A comparison of nystagmus habituation in the cat and dog. Acta Otolaryngol. 62:19–26.
Courjon JH, Precht W, Sirkin DW(1987) Vestibular nerve and nuclei unit responses and eye movement responses to repetitive galvanic stimulation of the labyrinth in the rat. Exp. Brain Res. 66:41–48.
Crampton GH (1962) Directional imbalance of vestibular nystagmus in cat following repeated unidirectional angular acceleration. Acta Otolaryngol. 55:41–48.
Crampton GH, Schwam WJ (1961) Effects of arousal reaction on nystagmus habituation in cat. Am. J. Physiol. 200:29–33.
Dow RS (1936) The fiber connections of the posterior parts of the cerebellum in the rat and cat. J. Comp. Neurol. 63:527–548.
Dow RS (1938) Efferent connections of the flocculo nodular lobe in macaca mulatta. J. Comp. Neurol. 68:297–305.
Dow ER, Anastasio TJ (1996) Violation of superposition by the vestibulo-ocular reflex of the goldfish. Neuroreport 7:1305–1309.
Fernández C, Fredrickson JM (1964) Experimental cerebellar lesions and their effect on vestibular function. Acta Otolaryngol. Suppl. 192:52–62.
Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE (1988) Frequency and velocity of rotational head perturbations during locomotion. Exp. Brain Res. 70:470–476.
Hartmann R, Klinke R (1980) Discharge properties of afferent fibres of the goldfish semicircular canal with high frequency stimulation. Pflügers Arch. 388:111–121.
Henriksson NG, Kohut R, Fernández C (1961) Studies on habituation of vestibular reflexes. Acta Otolaryng. 53:333–349.
Ito M, Shiida T, Yagi N, Yamamoto M (1974) Visual influence on rabbit horizontal vestibulo-ocular reflex presumably effected via the cerebellar flocculus. Brain Res. 65:170–174.
Ito J, Sasa M, Matsuoka I, Takaori S (1982) Afferent projection from reticular nuclei, inferior olive and cerebellum to lateral vestibular nucleus of the cat as demonstrated by horseradish peroxidase. Brain Res. 231:427–432.
Jäger J, Henn V (1981a) Habituation of the vestibulo-ocular reflex (VOR) in the monkey during sinusoidal rotation in the dark. Exp. Brain Res. 41:108–114.
Jäger J, Henn V (1981b) Vestibular habituation in man and monkey during sinusoidal rotation. In: B Cohen, ed. Vestibular and Oculomotor Physiology: International Meeting of the Bárány Society. New York Acadamy of Sciences, NewYork. vol. 374. pp. 330–339.
Jeannerod M, Clement G, Courjon JH, Schmid R (1981) Unilateral habituation of vestibulo-ocular responses in the cat. In: Cohen B, ed. Vestibular and Oculomotor Physiology: International Meeting of the Bárány Society. New York Acadamy of Sciences, New York. vol. 374. pp. 340–351.
Jeannerod M, Magnin M, Schmid R, Stefanelli M (1976) Vestibular habituation to angular velocity steps in the cat. Biol. Cybern. 22:39–48.
Kileny P, Ryu JH, McCabe BF, Abbas PJ (1980) Neuronal habituation in the vestibular nuclei of the cat. Acta Otolaryngol. 90:175–183.
Kleinschmidt HJ, Collewijn H (1975) A search for habituation of vestibulo-ocular reactions to rotary and linear sinusoidal accelerations in the rabbit. Exp. Neurol. 47:257–267.
Llinás RR, Walton KD (1990) Cerebellum. In: GM Shepherd, ed. The Synaptic Organization of the Brain. Oxford University Press, New York, pp. 214–245.
Mertens RA, Collins WE (1967) Unilateral caloric habituation of nystagmus in the cat: Effects on rotational and bilateral caloric responses. Acta Otolaryngol. 64:281–297.
Paige G. (1983) VOR and its interaction with visual following mechanisms in the squirrel monkey: I. Response characteristics in normal animals. J. Neurophysiol. 49:134–151.
Pastor AM, de la Cruz RR, Baker R (1992) Characterization and adaptive modification of the goldfish vestibulo-ocular reflex by sinusoidal and velocity step vestibular stimulation in the goldfish. J. Neurophysiol. 68:2003–2015.
Pastor AM, de la Cruz RR, Baker R (1994a) Cerebellar role in adaptation of the goldfish vestibuloocular reflex. J. Neurophysiol. 72:1383–1394.
Pastor AM, de la Cruz RR, Baker R (1994b) Eye position and eye velocity integrators reside in separate brainstem nuclei. Proc. Natl. Acad. Sci. USA 91:807–811.
Precht W, Volkind R, Maeda M, Giretti ML (1976) The effects of stimulating the cerebellar nodulus in the cat on the responses of vestibular neurons. Neurosci. 1:301–312.
Proctor LR, Fernández C (1963) Studies on habituation of vestibular reflexes: IV. Effect of caloric stimulation in blindfolded cats. Acta Otolaryngol. 56:500–508.
Remmel RS (1984) An inexpensive eye movement monitor using the scleral search coil technique. IEEE Trans. Biomed. Eng. 31:388–390.
Robinson DA (1963) A method for measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans. Biomed. Eng. 10:137–145.
Rumelhart DE, McClelland JL, P.D.P. Research Group (eds.) (1986) Parallel Distributed Processing: Explorations in the Microstructure of Cognition. Vol. 1, Foundations. MIT Press, Cambridge, MA.
Schairer JO, Bennett MVL (1986) Changes in gain of the vestibulo-ocular reflex induced by combined visual and vestibular stimulation in goldfish. Brain Res. 373:164–176.
Schmid R, Jeannerod M (1985) Vestibular habituation: An adaptive process? In: A Berthoz, G Melvill-Jones, eds. Adaptive Mechanisms in Gaze Control. Elsevier, New York. vol. 1, pp. 113–122.
Segal BN, Outerbridge JS (1982a) A nonlinear model of semicircular canal primary afferents in bullfrog. J. Neurophysiol. 47:563–578.
Segal BN, Outerbridge JS (1982b) Vestibular (semicircular canal) primary neurons in bullfrog: Nonlinearity of individual and population response to rotation. J. Neurophysiol. 47:545–562.
Shojaku H, Sato Y, Ikarashi K, Kawasaki T (1987) Topographical distribution of Purkinje cells in the uvula and the nodulus projecting to the vestibular nuclei in cats. Brain Res. 416:100–112.
Singleton GT (1967) Relationships of the cerebellar nodulus to vestibular function: A study of the effects of nodulectomy on habituation. Laryngoscope 77:1579–1620.
Solomon D, Cohen B (1994) Stimulation of the nodulus and uvula discharges velocity storage in the vestibular-ocular reflex. Exp. Brain Res. 102:57–68.
Tempia F, Dieringer N, Strata P (1991) Adaptation and habituation of the vestibulo-ocular reflex in intact and inferior olive-lesioned rats. Exp. Brain Res. 86:568–578.
Torte MP, Courjon JH, Flandrin JM, Magnin M, Magenes G (1994) Anatomical segregation of different adaptive processes within the vestibulocerebellum of the cat. Exp. Brain Res. 99:441–454.
Waespe W, Cohen B, Raphan T (1985) Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science 228:199–202.
Weissenstein L, Ratnam R, Anastasio TJ (1996) Vestibular compensation in the horizontal vestibulo-ocular reflex of the goldfish. Behav. Brain Res. 75:127–137.
Wilson V, Melvill Jones G (1979) Mammalian Vestibular Physiology, Plenum Press, New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dow, E.R., Anastasio, T.J. Analysis and Neural Network Modeling of the Nonlinear Correlates of Habituation in the Vestibulo-ocular Reflex. J Comput Neurosci 5, 171–190 (1998). https://doi.org/10.1023/A:1008818016900
Issue Date:
DOI: https://doi.org/10.1023/A:1008818016900