Abstract
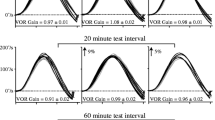
Modification of the vestibulo-ocular reflex (VOR) by vestibular habituation is an important paradigm in the study of neural plasticity. The VOR is responsible for rotating the eyes to maintain the direction of gaze during head rotation. The response of the VOR to sinusoidal rotation is quantified by its gain (eye rotational velocity/head rotational velocity) and phase difference (eye velocity phase—inverted head velocity phase). The frequency response of the VOR in naïve animals has been previously modeled as a high-pass filter (HPF). A HPF passes signals above its corner frequency with gain 1 and phase 0 but decreases gain and increases phase lead (positive phase difference) as signal frequency decreases below its corner frequency. Modification of the VOR by habituation occurs after prolonged low-frequency rotation in the dark. Habituation causes a reduction in low-frequency VOR gain and has been simulated by increasing the corner frequency of the HPF model. This decreases gain not only at the habituating frequency but further decreases gain at all frequencies below the new corner frequency. It also causes phase lead to increase at all frequencies below the new corner frequency (up to some asymptotic value). We show that habituation of the goldfish VOR is not a broad frequency phenomena but is frequency specific. A decrease in VOR gain is produced primarily at the habituating frequency, and there is an increase in phase lead at nearby higher frequencies and a decrease in phase lead at nearby lower frequencies (phase crossover). Both the phase crossover and the frequency specific gain decrease make it impossible to simulate habituation of the VOR simply by increasing the corner frequency of the HPF model. The simplest way to simulate our data is to subtract the output of a band-pass filter (BPF) from the output of the HPF model of the naïve VOR. A BPF passes signals over a limited frequency range only. A BPF decreases gain and imparts a phase lag and lead, respectively, as frequency increases and decreases outside this range. Our model produces both the specific decrease in gain at the habituating frequency, and the phase crossover centered on the frequency of habituation. Our results suggest that VOR habituation may be similar to VOR adaptation (in which VOR modification is produced by visual-vestibular mismatch) in that both are frequency-specific phenomena.
Similar content being viewed by others
References
Baker R, Evinger C, McCrea RA (1981) Some thoughts about the three neurons in the vestibular ocular reflex Ann. N.Y. Acad. Sci. 374: 171–188.
Baloh RW, Henn V, Jäger J (1982) Habituation of the human vestibulo-ocular reflex with low-frequency harmonic acceleration. Am. J. Otolaryngol. 3: 235–241.
Blair S, Gavin M (1979) Response of the vestibulo-ocular reflex to differing programs of acceleration. Invest. Ophthalmol. Visual Sci. 18: 1086–1090.
Buettner UW, Henn V, Young LR (1981) Frequency response of the vestibulo-ocular reflex (VOR) in the monkey. Aviat. Space Environ. Med. 52: 73–77.
Butler AB, Hodos W (1996) ComparativeVertebrate Neuroanatomy: Evolution and Adaptation. New York: Wiley.
Clément G, Courjon JH, Jeannerod M, Schmid R (1981) Unidirectional habituation of vestibulo-ocular responses by repeated rotational or optokinetic stimulations in the cat. Exp. Brain Res. 42: 34–42.
Cohen H, Cohen B, Raphan T, Waespe W (1992) Habituation and adaptation of the vestibuloocular reflex: A model of differential control by the vestibulocerebellum. Exp. Brain Res. 90: 526–538.
Collins WE (1964) Task-control of arousal and the effects of repeated unidirectional angular acceleration on human vestibular responses. Acta Otolaryngol. Suppl. 190: 1–34.
Collins WE, Updegraff BP (1965) A comparison of nystagmus habituation in the cat and dog. Acta Otolaryngol. 62: 19–26.
Dodge R (1923) Habituation to rotation. J. Exp. Psychol. 6: 1–35.
Dow ER, Anastasio TJ (1996) Violation of superposition by the vestibulo-ocular reflex of the goldfish. NeuroReport 7: 1305–1309.
Dow ER, Anastasio TJ (1997) Induction of periodic alternating nystagmus in intact gold-fish by sinusoidal rotation. NeuroReport 8: 2755–2759.
Dow ER, Anastasio TJ (1998a) Analysis and neural network modeling of the nonlinear correlates of habituation in the vestibuloocular reflex. J. Comput. Neuro. 5: 171–190.
Dow ER, Anastasio TJ (1998b) Instabilities in eyemovement control: A model of periodic alternating nystagmus. In: MI Jordan, MJ Kearns, SA Solla, eds. Advances in Neural Information Processing Systems 10. MIT Press, Cambridge. pp. 138–144.
Dow RS (1936) The fiber connections of the posterior parts of the cerebellum in the rat and cat. J. Comp. Neurol. 63: 527–548.
Dow RS (1938) Efferent connections of the flocculo nodular lobe in macaca mulatta. J. Comp. Neurol. 68: 297–305.
Godaux E, Halleux J, Gobert C (1983) Adaptive change of the vestibulo-ocular reflex in the cat: The effects of a long-term frequency-selective procedure. Exp. Brain Res. 49: 28–34.
Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE (1988) Frequency and velocity of rotational head perturbations during locomotion. Exp. Brain Res. 70: 470–476.
Henriksson NG, Kohut R, Fernández C (1961) Studies on habituation of vestibular reflexes. Acta Otolaryng. 53: 333–349.
Ito M, Takashi S, Nobuya Y, Mikyuki Y (1974) Visual influence on rabbit horizontal vestibulo-ocular reflex presumably effected via the cerebellar flocculus. Brain Res. 65: 170–174.
Jäger J, Henn V (1981a) Habituation of the vestibulo-ocular reflex (VOR) in the monkey during sinusoidal rotation in the dark. Exp. Brain Res. 41: 108–114.
Jäger J, Henn V (1981b) Vestibular habituation in man and monkey during sinusoidal rotation. In: B Cohen, ed. Vestibular and Oculomotor Physiology: International Meeting of the Bárány Society. New York Acadamy of Sciences, New York. Vol. 374, pp. 330–339.
Jeannerod M, Magnin M, Schmid R, Stefanelli M (1976) Vestibular habituation to angular velocity steps in the cat. Biol. Cybern. 22: 39–48.
Kleinschmidt HJ, Collewijn H (1975) A search for habituation of vestibulo-ocular reactions to rotary and linear sinusoidal accelerations in the rabbit. Exp. Neurol. 47: 257–267.
Llinás RR, Walton KD (1990) Cerebellum. In: GM Shepherd, ed. The Synaptic Organization of the Brain. Oxford University Press, New York. pp. 214–245.
Lisberger SG, Miles FA, Optican, LM (1983) Frequency-selective adaptation: Evidence for channels in the vestibulo-ocular reflex? J. Neurosci. 3: 1234–44.
Lisberger SG, Miles FA, Zee DS (1984) Signals used to compute errors in monkey vestibuloocular reflex: Possible role of flocculus. J. Neurophys. 52: 1140–1153.
Melvill Jones G (1985) Adaptive modulation of VOR parameters by vision. In: A Berthoz, Jones G Melvill, eds. Adaptive Mechanisms in Gaze Control. Elsevier, New York. pp. 21–50.
Miles FA, Optican LM, Lisberger SG (1985) An adaptive equalizer model of the primate vestibulo-ocular reflex. In: A Berthoz, Jones G Melvill, eds. Adaptive Mechanisms in Gaze Control. Elsevier, New York. pp. 313–326.
Milsum JH (1966) Biological Control Systems Analysis. McGraw-Hill, New York.
Pastor AM, de la Cruz RR, Baker R (1992) Characterization and adaptive modification of the goldfish vestibulo-ocular reflex by sinusoidal and velocity step vestibular stimulation in the goldfish. J. Neurophysiol. 68: 2003–2015.
Pastor AM, de la Cruz RR, Baker R (1994) Cerebellar role in adaptation of the goldfish vestibuloocular reflex. J. Neurophysiol. 72: 1383–1394.
Ratnam R, Anastasio TJ (1995) Evidence for a cooperative learning mechanism in the vestibulo-ocular reflex. NeuroReport 6: 2129–2133.
Raymond JL, Lisberger SG (1996) Behavioral analysis of signals that guide learned changes in the amplitude and dynamics of the vestibulo-ocular reflex. J. Neurosci. 16: 7791–7802.
Remmel RS (1984) An inexpensive eyemovement monitor using the scleral search coil technique. IEEE Trans. Biomed. Eng. 31: 388–390.
Robinson DA (1963) A method for measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans. Biomed. Eng. 10: 137–145.
Robinson DA (1976) Adaptive gain control of vestibuloocular reflex by the cerebellum. J. Neurophys. 39: 954–969.
Schairer JO, Bennett MVL (1986) Changes in gain of the vestibuloocular reflex induced by combined visual and vestibular stimulation in goldfish. Brain Res. 373: 164–176.
Schmid R, Jeannerod M (1985) Vestibular habituation: An adaptive process? In: A Berthoz and G. Melvill-Jones, eds. Adaptive Mechanisms in Gaze Control. Elsevier, NewYork.Vol. 1, pp. 113–122.
Singleton GT (1967) Relationships of the cerebellar nodulus to vestibular function: A study of the effects of nodulectomy on habituation. Laryngoscope 77: 1579–1620.
Smith PF, Curthoys IS (1989) Mechanisms of recovery following unilateral labyrinthectomy: Areview. Brain Res. Reviews 14: 155–180.
Torte MP, Courjon JH, Flandrin JM, Magnin M, Magenes G (1994) Anatomical segregation of different adaptive processes within the vestibulocerebellum of the cat. Exp. Brain Res. 99: 441–454.
Waespe W, Cohen B, Raphan T (1983) Role of the flocculus and paraflocculus in optokinetic nystagmus and visual-vestibular interactions: Effects of lesions. Exp. Brain Res. 50: 9–33.
Waespe W, Cohen B, Raphan T (1985) Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science 228: 199–202.
Weissenstein L, Ratnam R, Anastasio TJ (1996) Vestibular compensation in the horizontal vestibulo-ocular reflex of the goldfish. Behav. Brain. Res. 75: 127–137.
Wilson V, Melvill Jones G (1985) MammalianVestibular Physiology. Plenum Press, New York.
Zee DS, Yamazaki A, Butler PH, Gucer G (1981) Effects of ablation of flocculus and paraflocculus on eye movements in primate. J. Neurophysiol. 46: 878–899.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dow, E.R., Anastasio, T.J. Analysis and Modeling of Frequency-Specific Habituation of the Goldfish Vestibulo-Ocular Reflex. J Comput Neurosci 7, 55–70 (1999). https://doi.org/10.1023/A:1008967511172
Issue Date:
DOI: https://doi.org/10.1023/A:1008967511172