Abstract
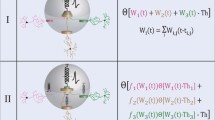
The classical view of cortical information processing is that of a bottom-up process in a feedforward hierarchy. However, psychophysical, anatomical, and physiological evidence suggests that top-down effects play a crucial role in the processing of input stimuli. Not much is known about the neural mechanisms underlying these effects. Here we investigate a physiologically inspired model of two reciprocally connected cortical areas. Each area receives bottom-up as well as top-down information. This information is integrated by a mechanism that exploits recent findings on somato-dendritic interactions. (1) This results in a burst signal that is robust in the context of noise in bottom-up signals. (2) Investigating the influence of additional top-down information, priming-like effects on the processing of bottom-up input can be demonstrated. (3) In accordance with recent physiological findings, interareal coupling in low-frequency ranges is characteristically enhanced by top-down mechanisms. The proposed scheme combines a qualitative influence of top-down directed signals on the temporal dynamics of neuronal activity with a limited effect on the mean firing rate of the targeted neurons. As it gives an account of the system properties on the cellular level, it is possible to derive several experimentally testable predictions.
Similar content being viewed by others
References
Adelson EH (1993) Perceptual organization and the judgment of brightness. Science 262:2042–2044.
Bar M, Ullman S (1996) Spatial context in recognition. Perception 25:343–352.
Budd JM (1998) Extrastriate feedback to primary visual cortex in primates: A quantitative analysis of connectivity. Proc. R. Soc. Lond. B. Biol. Sci. 265:1037–1044.
Buzsaki G, Kandel A (1998) Somadendritic backpropagation of action potentials in cortical pyramidal cells of the awake rat. J. Neurophysiol. 79:1587–1591.
Buzsaki G, Penttonen M, Nadasdy Z, Bragin A (1996) Pattern and inhibition-dependent invasion of pyramidal cell dendrites by fast spikes in the hippocampus in vivo. Proc. Natl. Acad. Sci. USA 93:9921–9925.
Carpenter G, Grossberg S (1987) A massively parallel architecture for a self-organizing neural pattern recognition machine. Comp. Vision Graphics Image Proc. 37:54–115.
Connors BW (1992) GABAA-and GABAB-mediated processes in visual cortex. Prog. Brain Res. 90:335–348.
Desimone R, Duncan J (1995) Neural mechanisms of selective visual attention. Ann. Rev. Neurosci. 18:193–222.
Downing CJ (1988) Expectancy and visual-spatial attention: Effects on perceptual quality. J. Exp. Psychol. Hum. Percept. Perform. 14:188–202.
Driver J, Spence C (1998) Cross-modal links in spatial attention. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 353:1319–1331.
Felleman DJ, Van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1:1–47.
Finkel LH, Edelman GM (1989) Integration of distributed cortical systems by reentry: A computer simulation of interactive functionally segregated visual areas. J. Neurosci. 9:3188–3208.
Goebel R, Khorram-Sefat D, Muckli L, Hacker H, Singer W (1998) The constructive nature of vision: Direct evidence from functional magnetic resonance imaging studies of apparent motion and motion imagery. Eur. J. Neurosci. 10:1563–1573.
Grossberg S (1980) How does a brain build a cognitive code? Psychol. Rev. 87:1–51.
Hupe JM, James AC, Payne BR, Lomber SG, Girard P, Bullier J (1998) Cortical feedback improves discrimination between figure and background by V1, V2 and V3 neurons. Nature 394:784–787.
Johnson RR, Burkhalter A (1997) A polysynaptic feedback circuit in rat visual cortex. J. Neurosci. 17:7129–7140.
Kim U, Sanchez-Vives MV, McCormick DA (1997) Functional dynamics of GABAergic inhibition in the thalamus. Science 278:130–134.
Koch C, Poggio T (1992) Multiplying with Synapses and Neurons. In: McKenna T, Davis J, Zornetzer SF, eds. Single Neuron Computation. Academic Press, San Diego. pp. 315–345.
König P, Luksch H (1998) Active sensing: Closing multiple loops. Z. Naturforsch. [C.] 53:542–549.
Kosslyn SM, Thompson WL, Kim IJ, Alpert NM (1995) Topographical representations of mental images in primary visual cortex. Nature 378:496–498.
Lamme VA (1995) The neurophysiology of figure-ground segregation in primary visual cortex. J. Neurosci. 15:1605–1615.
Lamme VA, Super H, Spekreijse H (1998) Feedforward, horizontal, and feedback processing in the visual cortex. Curr. Opin. Neurobiol. 8:529–535.
Larkum ME, Zhu JJ, Sakmann B (1999) A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature 3</del>98:338–341.
Lavie N, Driver J (1996) On the spatial extent of attention in object-based visual selection. Percept. Psychophys. 58:1238–1251.
Le Bihan D, Turner R, Zeffiro TA, Cuenod CA, Jezzard P, Bonnerot V (1993) Activation of human primary visual cortex during visual recall: A magnetic resonance imaging study. Proc. Natl. Acad. Sci. USA 90:11802–11805.
Lisman JE (1997) Bursts as a unit of neural information: Making unreliable synapses reliable. Trends. Neurosci. 20:38–43.
Livingstone MS, Freeman DC, Hubel DH (1996) Visual responses in V1 of freely viewing monkeys. Cold Spring Harb. Symp. Quant. Biol. 61:27–37.
Luck SJ, Chelazzi L, Hillyard SA, Desimone R (1997) Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J. Neurophysiol. 77:24–42.
Mignard M, Malpeli JG (1991) Paths of information flow through visual cortex. Science 251:1249–1251.
Moran J, Desimone R (1985) Selective attention gates visual processing in the extrastriate cortex. Science 229:782–784.
Mumford D (1992) On the computational architecture of the neocortex. II. The role of cortico-cortical loops. Biol. Cybern. 66:241–251.
Oram MW, Perrett DI (1994) Modeling visual recognition from neurobiological constraints. Neural Networks 7: 945–972.
Posner MI, Petersen SE (1990) The attention system of the human brain. Ann. Rev. Neurosci. 13:25–42.
Rao RPM (1999) An optimal estimation approach to visual perception and learning. Vision Res. 39:1963–1989.
Rao RPN, Ballard DH (1997) Dynamic model of visual recognition predicts neural response properties in the visual cortex. Neural Comp. 9:721–763.
Rockland KS, Virga A (1989) Terminal arbors of individual “feedback” axons projecting from area V2 to V1 in the macaque monkey: A study using immunohistochemistry of anterogradely transported Phaseolus vulgaris-leucoagglutinin. J. Comp. Neurol. 285:54–72.
Roelfsema PR, Lamme VA, Spekreijse H (1998) Object-based attention in the primary visual cortex of the macaque monkey. Nature 395:376–381.
Salin PA, Bullier J (1995) Corticocortical connections in the visual system: Structure and function. Physiol. Rev. 75:107–154.
Salin PA, Girard P, Bullier J (1993) Visuotopic organization of corticocortical connections in the visual system. Prog. Brain Res. 95:169–178.
Sandell JH, Schiller PH (1982) Effect of cooling area 18 on striate cortex cells in the squirrel monkey. J. Neurophysiol. 48:38–48.
Schiller J, Schiller Y, Stuart G, Sakmann B (1997) Calcium action potentials restricted to distal apical dendrites of rat neocortical pyramidal neurons. J. Physiol. (Lond.) 505:605–616.
Stins JF, van Leeuwen C (1993) Context influence on the perception of figures as conditional upon perceptual organization strategies. Percept. Psychophys. 53:34–42.
Stuart G, Schiller J, Sakmann B (1997a) Action potential initiation and propagation in rat neocortical pyramidal neurons. J. Physiol. (Lond.) 505:617–632.
Stuart G, Spruston N, Sakmann B, Hausser M (1997b) Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 20:125–131.
Stuart G, Sakmann B (1994) Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature 367:69–72.
Tsubokawa H, Ross WN (1997) Muscarinic modulation of spike backpropagation in the apical dendrites of hippocampal CA1 pyramidal neurons. J. Neurosci. 17:5782–5791.
Ullman S (1995) Sequence seeking and counter streams: A computational model for bidirectional information flow in the visual cortex. Cereb. Cortex. 5:1–11.
Victor JD, Mehler F, Reich D, Purpura K (1998) Spatiotemporal origin of bursts and “reliable” spikes generated by neurons in V1. Soc. Neurosci. (Abstract) 24:497.5.
von Stein A, Chiang C, König P (1998) Synchronization of activity between parietal cortex and primary visual cortex indicating the top-down processing of behaviorally significant stimuli. (submitted)
Watanabe T, Harner AM, Miyauchi S, Sasaki Y, Nielsen M, Palomo D, Mukai I (1998) Task-dependent influences of attention on the activation of human primary visual cortex. Proc. Natl. Acad. Sci. USA 95:11489–11492.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Siegel, M., Körding, K.P. & König, P. Integrating Top-Down and Bottom-Up Sensory Processing by Somato-Dendritic Interactions. J Comput Neurosci 8, 161–173 (2000). https://doi.org/10.1023/A:1008973215925
Issue Date:
DOI: https://doi.org/10.1023/A:1008973215925