Abstract
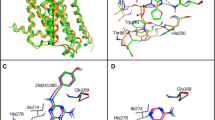
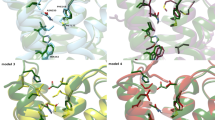
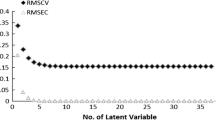
A set of 32 N6-substituted adenosines and 22 8-substituted xanthines with affinity for adenosine A1 receptors was subjected to three-dimensional quantitative structure-affinity relationship analysis using comparative molecular field analysis (CoMFA). The aim was to compare two modes of binding to the receptor – `N6-C8' and `N6-N7'. Good models with high predictive power and stability were obtained. A comparison of these models gives the following results: (a) Inclusion of both steric and electrostatic fields in CoMFA generates better predictive models compared to models based on steric or electrostatic fields alone. (b) The `N6-N7' CoMFA models are slightly better than the `N6-C8' ones. (c) Steric restriction exists around the N6-H in the `N6-N7' steric field map, which is absent in the `N6-C8' steric field map. This report demonstrates that the `N6-N7' mode of binding is a further development of the `N6-C8' model with a slightly better predictive ability and more accurate steric and electrostatic overlaps between agonists and antagonists.
Similar content being viewed by others
References
Libert, F., Schiftmann, S.N., Lefort, A., Partmentier, M., Gerard, C., Dumont, J.E., Vanderhaeghen, J.-J. and Vassart, G., EMBO J., 10 (1991) 1677.
van Galen, P.J.M., van Vlijmen, H.W.Th., IJzerman, A.P. and Soudijn, W., J. Med. Chem., 33 (1990) 1708.
Peet, N.P., Lentz, N.L., Meng, E.C., Dudley, M.V., Ogden, A.M.L., Demeter, D.A., Weintraub, H.J.R. and Bey, P., J. Med. Chem., 33 (1990) 3127.
van der Wenden, E.M., IJzerman, A.P. and Soudijn, W., J. Med. Chem., 35 (1992) 629.
Doytchinova, I. and Petrova, S., Med. Chem. Res., 8 (1998) 143.
Pajeva, I. and Wiese, M., J. Med. Chem., 41 (1998) 1815.
Sadler, B.R., Cho, S.J., Ishaq, K.S., Chae, K. and Korach, K.S., J. Med. Chem., 41 (1998) 2261.
Mor, M., Rivava, S., Silva, C., Bordi, F. and Plazzi, V., J. Med. Chem., 41 (1998) 3831.
Trivedi, B.K. and Bruns, R.F., J. Med. Chem., 32 (1989) 1667.
Jacobson, K.A., van Galen, P.J.M. and Williams, M., J. Med. Chem., 35 (1992) 407.
Trivedi, B.K., Blankley, C.J., Bristol, J.A., Hamilton, H.W., Patt, W.C., Kramer, W.J., Johnson, S.A., Bruns, R.F., Cohen, D.M. and Ryan, M.J., J. Med. Chem., 34 (1991) 1043.
Shamim, M.T., Ukena, D., Padgett, W.L. and Daly, J.W., J. Med. Chem., 32 (1989) 1231.
Shamim, M.T., Ukena, D., Padgett, W.L., Hong, O. and Daly, J.W., J. Med. Chem., 31 (1988) 613.
van der Wenden, E.M., van Galen, P.J.M., IJzerman, A.P. and Soudijn, W., J. Med. Str. (Theochem), 231 (1991) 175.
Chem-X (July 1998). Chemical Design Ltd., Chipping Norton, Oxfordshire OX7 5SR, U.K.
Burkert, U. and Allinger, N.L., Molecular Mechanics, Am. Chem. Soc., Washington, DC, 1982.
Dewar, M.J.S., Zoebisch, E.G., Healy, E.F. and Stewart, J.J.P., J. Am. Chem. Soc., 107 (1985) 3902.
Dunn, W.J., III, Wold, S., Edlund, U., Hellberg, S. and Gasteiger, J., Quant. Struct.-Act. Relat., 3 (1984) 131.
Wold, S., In Van de Waterbeemd, H. (Ed.) Chemometric Methods in Molecular Design, VCH, Weinheim, 1995, pp 195-218.
Mueller, Chr., Exp. Opin. Ther. Patents, 7 (1997) 419.
Bruns, R.F., Lu, G.H. and Pugsley, T.A., Mol. Pharmacol., 29 (1986) 331.
Lohse, M.J., Klotz, K.-N., Schwabe, U., Cristalli, G., Vittori, S. and Grifantini, M., Naunyn-Schmiedeberg's Arch. Pharmacol., 29 (1988) 687.
Nair, V. and Fasbender, A.J., Tetrahedron, 49 (1993) 2169.
Grahner, B., Winiwarter, S., Lanzner, W. and Muller, C.E., J. Med. Chem., 37 (1994) 1526.
IJzerman, A.P., van Galen, P.J.M. and Jacobson, K.A., Drug Des. Discov., 9 (1992) 49.
Dudley, M.W., Peet, N.P., Demeter, D.A., Weintraub, H.J.R., IJzerman, A.P., Nordvall, G., van Galen, P.J.M. and Jacobson, K.A., Drug Dev. Res., 28 (1993) 237.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Doytchinova, I. CoMFA-based comparison of two models of binding site on adenosine A1 receptor. J Comput Aided Mol Des 15, 29–39 (2001). https://doi.org/10.1023/A:1011150120831
Issue Date:
DOI: https://doi.org/10.1023/A:1011150120831