Abstract
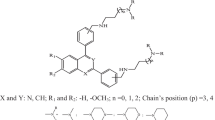
A set of 18 structurally diverse antifolates including pyrimethamine, cycloguanil, methotrexate, aminopterin and trimethoprim, and 13 pyrrolo[2,3-d]pyrimidines were studied using four-dimensional quantitative structure-activity relationship (4D-QSAR) analysis. The corresponding biological activities of these compounds include IC50 inhibition constants for both the wild type, and a specific mutant type of Plasmodium falciparum dihydrofolate reductase (DHFR). Two thousand conformations of each analog were sampled to generate a conformational ensemble profile (CEP) from a molecular dynamics simulation (MDS) of 100,000 conformer trajectory states. Each sampled conformation was placed in a 1 Å cubic grid cell lattice for each of five trial alignments. The frequency of occupation of each grid cell was computed for each of six types of pharmacophore groups of atoms of each compound. These grid cell occupancy descriptors (GCODs) were then used as a descriptor pool to construct 4D-QSAR models. Models for inhibition of both the `wild' type and the mutant enzyme were generated which provide detailed spatial pharmacophore requirements for inhibition in terms of atom types and their corresponding relative locations in space. The 4D-QSAR models indicate some structural features perhaps relevant to the mechanism of resistance of the Plasmodium falciparum DHFR to current antimalarials. One feature identified is a slightly different binding alignment of the ligands to the mutant form of the enzyme as compared to the wild type.
Similar content being viewed by others
References
The World Health Organization Report; Who Publications, Geneva, 1997.
Blakley, R.L., In: Blakley, R.L. and Benkovic, S.J. (Eds) Folates and Pterins, Vol. 1; John Wiley & Sons, New York, NY, 1984, p. 191.
Brown, K.A. and Kraut, J., Faraday Discuss., 93 (1992) 217.
Kraut, J. and Matthews, D.A., In Jurnak, F. and McPherson, A. (Eds), Biological Macromolecules and Assemblies, Vol. 3. John Wiley & Sons, New York, NY, 1987, p. 1.
Miller, G.P. and Benkovic, S.J., J. Chem. Biol., 5 (1998) R105.
Bzik, D.J., Li, W.-B., Horii, T. and Inserburg, J., Proc. Natl. Acad. Sci. USA, 84 (1987) 8360.
Basco, L.K., DePécoulas, P.E., Wilson, C.M., LeBras, J. and Mazabraud, A., Mol. Biochem. Parasitol., 69 (1998) 135.
Cowman, A.F., Morry, M.J., Biggs, B.A., Cross, G.A.M. and Foot, S.J., Proc. Natl. Acad. Sci. USA, 85 (1988) 9109.
Peterson, D.S., Walliker, D. and Wellens, T.E., Proc. Natl. Acad. Sci. USA, 85 (1998) 9114.
Peterson, D.S., DiSanti, S.M., Povoa, M., Calvosa, V.S., DoRosario, V.E. and Wellens, T.E., Am. J. Trop. Med. Hyg., 45 (1991) 492.
Peterson, D.S., Milhouse, W.K. and Wellens, T.E., Proc. Natl. Acad. Sci. USA, 87 (1990) 3018.
Thaithong, S., Chan, S.-W., Songsomboon, S., Wilairat, P., Seesod, N., Sueblinwong, T., Goman, M., Ridley, R. and Beale, G., Mol. Biochem. Parasitol., 52 (1992) 149.
Snewin, V.A., England, S.M., Sims, P.F.G. and Hyde, J.E., Gene, 76 (1989) 41.
Foot, S.J., Galatis, D. and Cowman, A.F., Proc. Natl. Acad. Sci. USA, 87 (1990) 3014.
Brobey, R.K.B., Iwakura, M., Itoh, F., Aso, K. and Horii, T., Parasitol. Int., 47 (1998) 69.
Hopfinger, A.J., Wang, S., Tokarski, J.S., Jin, B., Albuquerque, M.G., Madhav, P.J. and Duraiswami, C., J. Am. Chem. Soc., 119 (1997) 10509.
van Gunsteren, W.F. and Berendsen, H.J.C., Angew. Chem., Int. Ed. Engl., 29 (1990) 992.
HyperChem Program Release 5.01 for Windows; Hypercube, Inc., 1996.
Dewar, M.J.S. and Theil, W., J. Am. Chem. Soc., 99 (1977) 4899.
Molsim User's Guide v.3.0, Molecular Mechanics and Dynamics Simulation Software, D.C. Doherty and The Chem21 Group, Inc., Lake Forest, IL, 1994.
Sano, G.-I., Morimatsu, K. and Horii, T., Mol. Biochem. Parasitol., 63 (1994) 265.
Glen, W.G., Dunn, W.J., III and Scott, D.R., Tetrahedron Comput. Methods, 2 (1989) 349.
Holland, J., Adaptation in Artificial and Natural Systems. University of Michigan Press, Ann Arbor, MI, 1975.
Rogers, D. and Hopfinger, A.J., J. Chem. Inf. Comput. Sci., 34 (1994) 854.
Rogers, D., WOLF Reference Manual Version 5.5, Molecular Simulation Inc., 1994.
Friedman, J., Multivariate Adaptative Regression Splines. Technical Report No. 102; Laboratory for Computational Statistics, Department of Statistics, Stanford University, Stanford, CA, November “1988 (revised August 1990).
4D-QSAR Manual v.1.0, A.J. Hopfinger and The Chem21 Group, Inc., Lake Forest, IL, 1997.
Tokarski, J.S. and Hopfinger, A.J., J. Chem. Inf. Comput. Sci., 37 (1997) 779.
Tokarski, J.S. and Hopfinger, A.J., J. Chem. Inf. Comput. Sci., 37 (1997) 792.
Peterson, M.R., Hall, D.R., Berriman, M., Nunes, J.A., Leonard, G.A., Fairlamb, A.H. and Hunter, W.N., J. Mol. Biol., 298 (2000) 1230.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Santos-Filho, O.A., Hopfinger, A.J. A search for sources of drug resistance by the 4D-QSAR analysis of a set of antimalarial dihydrofolate reductase inhibitors. J Comput Aided Mol Des 15, 1–12 (2001). https://doi.org/10.1023/A:1011152818340
Issue Date:
DOI: https://doi.org/10.1023/A:1011152818340