Abstract
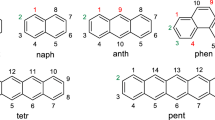
A new topological charge-transfer index is applied toelucidate the polar character of hydrocarbons. The dipole moments calculated by algebraic and vector semisums of charge-transfer indices are defined in this work. The combination of the charge-transfer indices allows the estimation of the molecular dipole moments. The ability of the indices for the description of the molecular charge distribution is established by comparing them with the dipole moment of a heterogeneous set of 57 polar and 53 apolar hydrocarbons. Linear and nonlinear correlation models are obtained. The new charge-transfer index, μvec, elucidates whether hydrocarbons are apolar, and improves the multivariable nonlinear regression equation for the dipole moment. When comparing with previous results, smaller superpositions of the corresponding pairs G k, J k are observed in our fits. This diminishes the risk of colinearity.
Similar content being viewed by others
References
Balaban, A.T. (ed.), Chemical Applications of Graph Theory. Academic Press, London, 1976.
Kier, L.B. and Hall, L.H., Molecular Connectivity in Chemistry and Drug Research. Academic Press, New York, NY, 1976.
Randić, M., Krais, G.A. and Deonauv-Jerman-Blazic, B., Chemical Applications of Topology and Graph Theory. Elsevier, Amsterdam, 1983, p. 192.
Trinajstić, N., Chemical Graph Theory. CRC, Boca Raton, FL, 1983.
Klopman, G., J. Am. Chem. Soc., 106 (1984) 7315-7321.
Kier, L.B. and Hall, L.H., Molecular Connectivity in Structure-Activity Analysis. Research Studies, Letchworth, 1986, p. 69.
Balaban, A.T., in Berthier, G., Dewar, M.J.S., Fischer, H., Fukui, K., Hartmann, H., Jaffé, H.H., Jortner, J., Kutzelnigg, W., Ruedenberg, K., Scrocco, E. and Zeil, W. (eds.), Steric Fit in Quantitative Structure-Activity Relations Lecture Notes in Chemistry, No. 15, Springer-Verlag, Berlin, 1980, Chapter 3.
Wiener, H., J. Am. Chem. Soc., 69 (1947) 17-20.
Mihalić, Z., Nikolić, S. and Trinajstić, N., J. Chem. Inf. Comput. Sci., 32 (1992) 28-37.
Harary, F., Graph Theory. Addison-Wesley, Reading, 1969.
Wilson, R., Introduction to Graph Theory. Academic Press, New York, NY 1972.
Busacker R.G. and Saaty T.L., Finite Graphs and Networks. McGraw-Hill, New York, NY, 1965.
Rouvray, D.H. and Balaban, A.T., in Wilson, R.J. and Beineke L.W. (eds.), Applications of Graph Theory, Academic Press, London, 1979.
Rouvray, D.H., RIC Rev., 4 (1971) 173-195.
Rouvray, D.H., Chem. Br., 10 (1974) 11-15.
Rouvray, D.H., J. Chem. Educ., 52 (1975) 768-773.
Ruedenberg, K., J. Chem. Phys., 22 (1954) 1878-1894.
Rouvray, D.H., in Balaban, A.T. (ed.), Chemical Application of Graph Theory, Academic Press, London, 1972, Chapter 7.
Graovac, A., Gutman, I. and Trinajstić, N., Topological Approach to the Chemistry of Conjugated Molecules. Lecture Notes in Chemistry, No. 4. Springer-Verlag, Berlin, 1976.
Rouvray, D.H., Am. Sci., 61 (1973) 729-735.
Rouvray, D.H., MATCH, 1 (1975) 125-134.
Randić, M., J. Am. Chem. Soc., 97 (1975) 6609-6615.
Murray, W.J., Kier, L.B. and Hall, L.H., J. Pharm. Sci., 64 (1975) 1978-1981.
Balaban, A.T., J. Chem. Inf. Comput. Sci., 32 (1992) 23-28.
Hosoya, H., Bull. Chem. Soc. Jpn., 44 (1971) 2332-2339.
Shifrin, S., in Cavallito C.J. (ed.), Structure-Activity Relationships. International Encyclopedia of Pharmacology and Therapeutics, Section 5, No. 1. Pergamon Press, Oxford, 1973, Chapter 4.
Gálvez, J., García, R., Salabert, M.T. and Soler, R., J. Chem. Inf. Comput. Sci., 34 (1994) 520-525.
Gálvez, J., García-Doménech, R., de Julián-Ortiz, J.V. and Soler, R., J. Chem. Inf. Comput. Sci., 35 (1995) 272-284.
Gálvez, J., García-Doménech, R., de Gregorio-Alapont, C., de Julián-Ortiz, J.V. and Popa, L., J. Mol. Graphics, 14 (1996) 272-276.
Ponce, A.M., Blanco, S.E., Molina, A.S., García-Domenech, R. and Gálvez, J., J. Chem. Inf. Comput. Sci., 40 (2000) 1039-1045.
De Julián-Ortiz, J.V., García-Doménech, R., Gálvez-Alvarez, J., Soler-Roca, R., García-March, F.J. and Antón-Fos, G.M., J. Chromatogr. A, 719 (1996) 37-44.
De Julián-Ortiz, J.V., de Gregorio-Alapont, C., Ríos-Santamarina, I., García-Doménech, R. and Gálvez, J., J. Mol. Graphics Mod., 16 (1998) 14-18.
Dewar, M.J.S., Zoebisch, E.G., Healy, E.F., Stewart, J.J.P., J. Am. Chem. Soc., 107 (1985) 3902-3909.
Torrens, F., Sánchez-Marín, J. and Nebot-Gil, I. J. Mol. Struct. (Theochem), 463 (1999) 27-39.
Hocking, R.R. Biometrics, 32 (1976) 1.
McClellan, A.L., Tables of Experimental Dipole Moments. Freeman, San Francisco, 1963.
Handbook of Tables of Organic Compounds Identification. CRC, Cleveland.
Tasi, G. and Mizukami, F., J. Chem. Inf. Comput. Sci., 38 (1998) 632-638.
Serrano-Andrés, L., Roos, B.O. and Merchán, M., Theor. Chim. Acta (Berlin), 87 (1994) 387-402.
Roos, B.O., Merchán, M., McDiarmid, R. and Xing, X., J. Am. Chem. Soc., 116 (1994) 5927-5936.
Molina, V., Smith, B.R., Merchán, M.A., Chem. Phys. Lett., 309 (1999), 486-494.
Molina, V., Merchán, M. and Roos, B.O., Spectrochim. Acta, A55 (1999) 433-446.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Torrens, F. A new topological index to elucidate apolar hydrocarbons. J Comput Aided Mol Des 15, 709–719 (2001). https://doi.org/10.1023/A:1012214227098
Issue Date:
DOI: https://doi.org/10.1023/A:1012214227098