Abstract
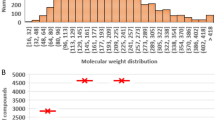
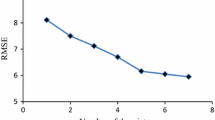
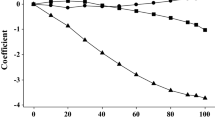
It has been shown that water solubility and octanol/water partition coefficient for a large diverse set of compounds can be predicted simultaneously using molecular descriptors derived solely from a two dimensional representation of molecular structure. These properties have been modelled using multiple linear regression, artificial neural networks and a statistical method known as canonical correlation analysis. The neural networks give slightly better models both in terms of fitting and prediction presumably due to the fact that they include non-linear terms. The statistical methods, on the other hand, provide information concerning the explanation of variance and allow easy interrogation of the models. Models were fitted using a training set of 552 compounds, a validation set and test set each containing 68 molecules and two separate literature test sets for solubility and partition.
Similar content being viewed by others
References
Livingstone, D.J., J.Chem. Inf. Comput. Sci., 40 (2000) 195.
Nirmalakhandan, N.N. and Speece, R.E., Environ. Sci. Technol., 22 (1988) 328.
Bodor, N. and Huang, M-J., J. Pharm. Sci., 81 (1992) 954.
Patil, G.S., J. Hazard. Mater., 36 (1994) 35.
Sutter, J.M. and Jurs, P.C., J. Chem. Inf. Comput. Sci., 36 (1996) 100.
Huibers, P.D.T. and Katritzky, A.R., J.Chem.Inf.Comput.Sci., 38 (1998) 283.
Mitchell, B.E. and Jurs, P.C., J. Chem. Inf. Comput. Sci., 38 (1998) 489.
Rekker, R. E., Hydrophobic Fragment Constant; Elsevier: New York, 1977.
Hansch, C. and Leo, A., Substituent Constants for Correlation Analysis in Chemistry and Biology; Wiley: New York, 1979.
Klopman, G. and Iroff, L., J. Comput. Chem., 2 (1981) 157.
Bodor, N. and Huang, M-J., J. Pharm. Sci., 81 (1992) 272.
Leo, A., Chem. Rev., 93 (1993) 1281.
Klopman, G.; Li, J-Y.; Wang, S. and Dimayuga, M., J. Chem. Inf. Comput. Sci., 34 (1994) 752.
Meylan, W. M. and Howard, P. H., J. Pharm. Sci., 84 (1995) 83.
Wang, R.; Fu, Y. and Lai, L., J. Chem. Inf. Comput. Sci. 37 (1997) 615.
Haeberlin, M. and Brinck, T., J. Chem. Soc. Perkin Trans. 2, (1997) 289.
Bodor, N. and Buchwald, P., J. Phys. Chem., 101 (1997) 3404.
Buchwald, P. and Bodor, N., Current. Med. Chem., 5 (1998) 353.
Bodor, N. and Huang, M-J., J.Am.Chem.Soc., 113 (1991) 9480.
Breindl, A.; Beck, N.; Clark, T. and Glen, R. C., J. Mol. Model., 3 (1997) 142.
Schaper, K.-J. and Samitier, M. L. R., Quant. Struct.-Act. Relat. 16 (1997) 224.
Devillers, J.; Domine, D. and Guillon, C., Eur. J. Med. Chem. 33 (1998) 659.
Huuskonen, J. J.; Salo, M. and Taskinen, J., J. Pharm. Sci. 86 (1997) 450.
Huuskonen, J. J.; Salo, M. and Taskinen, J., J. Chem. Inf. Comput. Sci., 38 (1998) 450.
Huuskonen, J.J., Rantanen, J. and Livingstone, D., Eur. J. Med. Chem., 35 (2000) 1081.
Huuskonen, J. J., J. Chem. Inf. Comput. Sci., 40 (2000) 773.
Huuskonen, J. J.; Villa, A. E. P. and Tetko, I. V., J. Pharm. Sci. 88 (1999) 229.
Huuskonen, J. J.; Livingstone, D.J. and Tetko, I. V., J. Chem. Inf. Comput. Sci., 40 (2000) 947.
Hall, L.H. and Kier, L.B., J. Chem. Inf. Comput. Sci. 35 (1995) 1039.
Szydlo, R.M., Ford M.G., Greenwood, R.G. and Salt, D.W., In Dearden, J.C. (ed.), Quantitative Approaches to Drug Design, Elsevier, Amsterdam, 1983, pp. 203-14.
Laass, W., In Seydel, J.K. (ed.), QSAR and Strategies in the Design of Bioactive Compounds, VCH, Weinheim, 1985, pp. 285-289.
Bordas, B., In Seydel, J.K. (ed.), QSAR and Strategies in the Design of Bioactive Compounds, VCH, Weinheim, 1985, pp. 389-392.
Ford, M.G. and Salt, D.W., In van de Waterbeemd, H. (ed.), Chemometric Methods in Molecular Design, VCH, Weinheim, 1995, pp. 265-282.
Yalkowsky, S.H. and Dannenfelser, R-M. (ed.), AQUASOL dATAbASE of Aqueous Solubility, College of Pharmacy, University of Arizona, Arizona, USA, 1990.
Biobyte, Corp., 201W. Fourth St., Suite #204, Claremont, CA 91711, USA.
Moriguchi, I., Hirono, S., Nakagome, I. And Hirono, H. Chem. Pharm. Bull., 42 (1994) 976-978.
Yalkowsky, S., Chemosphere 26 (1993) 1239-1261.
BMDP Statistical Software Manual, Dixon, W.J. (ed.) University of California Press, 1990.
Tetko, I.V., Villa, A.E.P. and Livingstone, D.J., J. Chem. Inf. Comput. Sci, 36 (1996) 794.
Wikel, J.H. and Dow, E.R., BioMed. Chem. Lett. 3 (1993) 645.
Kühne, R., Ebert, R.-U., Kleint, F., Scmidt, G. and Schüürmann, G. Chemosphere 30 (1995) 2061
Klopman, G., Wang, S., Balthasar, D.M., J.Chem.Inf.Comput.Sci. 32 (1992) 474
Stewart, D. K. and Love, W. A. Psychol. Bull., 70 (1968) 160.
Roadknight, C.M., Palmer-Brown, D. and Mills, G.E., In Liu, X., Cohen, P. and Berthold, M. (eds), Advances in Intelligent Data Analysis, Springer-Verlag, Berlin, 1997, pp. 337-346.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Livingstone, D.J., Ford, M.G., Huuskonen, J.J. et al. Simultaneous prediction of aqueous solubility and octanol/water partition coefficient based on descriptors derived from molecular structure. J Comput Aided Mol Des 15, 741–752 (2001). https://doi.org/10.1023/A:1012284411691
Issue Date:
DOI: https://doi.org/10.1023/A:1012284411691